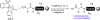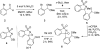A reagent for the convenient, solid-phase synthesis of N-terminal peptide hydroxylamines for chemoselective ligations
- PMID: 19256487
- PMCID: PMC2677186
- DOI: 10.1021/ja900601c
A reagent for the convenient, solid-phase synthesis of N-terminal peptide hydroxylamines for chemoselective ligations
Abstract
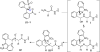
A new N-sulfonyloxaziridine reagent for the conversion of N-terminal peptide amines to the corresponding hydroxylamines without overoxidation or erosion of stereochemistry is described. The products are N-terminal hydroxylamines, which are substrates for chemoselective amide-bond forming reactions with alpha-ketoacids. The success of this reagent arises from covalent precomplexation with the substrate via imine bond formation followed by an intramolecular oxygen-atom transfer to the amine. An unexpected stereochemical mismatch between the racemic reagent and the chiral peptide substrates was addressed by the preparation of an enantipure version of this reagent and provides further support for the proposed mechanism.
Figures
References
-
- Rosenblatt DH, Burrows EP. In: Supplement F: The Chemistry of Amino, Nitroso, and Nitro Compounds and Their Derivatives. Part 2. Patai S, editor. Wiley and Sons; Chichester: 1982. pp. 1085–1149.
-
- Field JD, Kropp PJ. J. Org. Chem. 2000;65:5937–5941. - PubMed
- Wittman MD, Halcomb RL, Danishefsky SJ. J. Org. Chem. 1990;55:1981–1983.
-
- Fennell KA, Miller MJ. J. Org. Chem. 1999;64:7451–7458.
- John ORS, Killeen NM, Knowles DA, Yau SC, Bagley MC, Tomkinson NCO. Org. Lett. 2007;9:4009–4012. - PubMed
-
- Bode JW, Fox RM, Baucom KD. Angew. Chem. Int. Ed. 2006;45:1248–1252. - PubMed
-
- Hackenberger CPR, Schwarzer D. Angew. Chem. Int. Ed. 2008;47:10030–10074. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources