Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin
- PMID: 19256547
- PMCID: PMC2748423
- DOI: 10.1021/ja809217u
Determining the effects of lipophilic drugs on membrane structure by solid-state NMR spectroscopy: the case of the antioxidant curcumin
Abstract
Curcumin is the active ingredient of turmeric powder, a natural spice used for generations in traditional medicines. Curcumin's broad spectrum of antioxidant, anticarcinogenic, antimutagenic, and anti-inflammatory properties makes it particularly interesting for the development of pharmaceutical compounds. Because of curcumin's various effects on the function of numerous unrelated membrane proteins, it has been suggested that it affects the properties of the bilayer itself. However, a detailed atomic-level study of the interaction of curcumin with membranes has not been attempted. A combination of solid-state NMR and differential scanning calorimetry experiments shows curcumin has a strong effect on membrane structure at low concentrations. Curcumin inserts deep into the membrane in a transbilayer orientation, anchored by hydrogen bonding to the phosphate group of lipids in a manner analogous to cholesterol. Like cholesterol, curcumin induces segmental ordering in the membrane. Analysis of the concentration dependence of the order parameter profile derived from NMR results suggests curcumin forms higher order oligomeric structures in the membrane that span and likely thin the bilayer. Curcumin promotes the formation of the highly curved inverted hexagonal phase, which may influence exocytotic and membrane fusion processes within the cell. The experiments outlined here show promise for understanding the action of other drugs such as capsaicin in which drug-induced alterations of membrane structure have strong pharmacological effects.
Figures
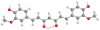





References
-
- Joe B, Vijaykumar M, Lokesh BR. Crit Rev Food Sci Nutr. 2004;44:97–111. - PubMed
-
- Hafner-Bratkovic I, Gaspersic J, Smid LM, Bresjanac M, Jerala R. J Neurochem. 2008;104:1553–1564. - PubMed
-
- Ono K, Hasegawa K, Naiki H, Yamada M. J Neurosci Res. 2004;75:742–750. - PubMed
-
- Reinke AA, Gestwicki JE. Chem Biol Drug Des. 2007;70:206–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

