Addition of a sequence from alpha2-antiplasmin transforms human serum albumin into a blood clot component that speeds clot lysis
- PMID: 19257897
- PMCID: PMC2654442
- DOI: 10.1186/1472-6750-9-15
Addition of a sequence from alpha2-antiplasmin transforms human serum albumin into a blood clot component that speeds clot lysis
Abstract
Background: The plasma protein alpha2-antiplasmin (alpha2AP) is cross-linked to fibrin in blood clots by the transglutaminase factor XIIIa, and in that location retards clot lysis. Competition for this effect could be clinically useful in patients with thrombosis. We hypothesized that fusion of N-terminal portions of alpha2-antiplasmin to human serum albumin (HSA) and production of the chimeric proteins in Pichia pastoris yeast would produce a stable and effective competitor protein.
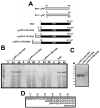
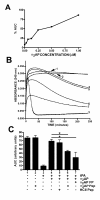
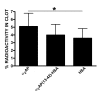
Results: Fusion protein alpha2AP(13-42)-HSA was efficiently secreted from transformed yeast and purified preparations contained within a mixed population the full-length intact form, while fusions with longer alpha2AP moieties were inefficiently secreted and/or degraded. The alpha2AP(13-42)-HSA protein, but not recombinant HSA, was cross-linked to both chemical lysine donors and fibrin or fibrinogen by factor XIIIa, although with less rapid kinetics than native alpha2AP. Excess alpha2AP(13-42)-HSA competed with alpha2AP for cross-linking to chemical lysine donors more effectively than a synthetic alpha2AP(13-42) peptide, and reduced the alpha2AP-dependent resistance to fibrinolysis of plasma clots equally effectively as the peptide. Native alpha2AP was found in in vivo clots in rabbits to a greater extent than alpha2AP(13-42), however.
Conclusion: In this first report of transfer of transglutamination substrate status from one plasma protein to another, fusion protein alpha2AP(13-42)-HSA was shown to satisfy initial requirements for a long-lasting, well-tolerated competitive inhibitor of alpha2-antiplasmin predicted to act in a clot-localized manner.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

