Identification of GRO1 as a critical determinant for mutant p53 gain of function
- PMID: 19258312
- PMCID: PMC2673286
- DOI: 10.1074/jbc.M900994200
Identification of GRO1 as a critical determinant for mutant p53 gain of function
Abstract
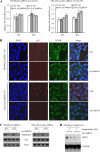
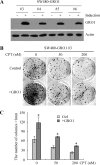
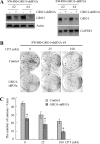
Mutant p53 gain of function contributes to cancer progression, increased invasion and metastasis potentials, and resistance to anticancer therapy. The ability of mutant p53 to acquire its gain of function is shown to correlate with increased expression of progrowth genes, such as c-MYC, MDR1, and NF-kappaB2. However, most of the published studies to identify mutant p53 target genes were performed in a cell system that artificially overexpresses mutant p53. Thus, it remains unclear whether such mutant p53 targets can be regulated by endogenous physiological levels of mutant p53. Here, we utilized SW480 and MIA-PaCa-2 cells, in which endogenous mutant p53 can be inducibly knocked down, to identify mutant p53 target genes that potentially mediate mutant p53 gain of function. We found that knockdown of mutant p53 inhibits GRO1 expression, whereas ectopic expression of mutant R175H in p53-null HCT116 cells increases GRO1 expression. In addition, we found that endogenous mutant p53 is capable of binding to and activating the GRO1 promoter. Interestingly, ectopic expression of GRO1 can rescue the proliferative defect in SW480 and MIA-PaCa-2 cells induced by knockdown of mutant p53. Conversely, knockdown of endogenous GRO1 inhibits cell proliferation and thus abrogates mutant p53 gain of function in SW480 cells. Taken together, our findings define a novel mechanism by which mutant p53 acquires its gain of function via transactivating the GRO1 gene in cancer cells. Thus, targeting GRO1 for cancer therapy would be applicable to a large portion of human tumors with mutant p53, but the exploration of GRO1 as a potential target should take the mutation status of p53 into consideration.
Figures

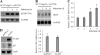
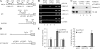

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

