NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury
- PMID: 19258328
- PMCID: PMC2676006
- DOI: 10.1074/jbc.M806084200
NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury
Abstract
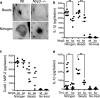
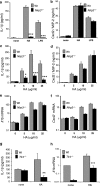
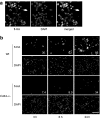
Inflammation under sterile conditions is a key event in autoimmunity and following trauma. Hyaluronan, a glycosaminoglycan released from the extracellular matrix after injury, acts as an endogenous signal of trauma and can trigger chemokine release in injured tissue. Here, we investigated whether NLRP3/cryopyrin, a component of the inflammasome, participates in the inflammatory response to injury or the cytokine response to hyaluronan. Mice with a targeted deletion in cryopyrin showed a normal increase in Cxcl2 in response to sterile injuries but had decreased inflammation and release of interleukin-1beta (IL-1beta). Similarly, the addition of hyaluronan to macrophages derived from cryopyrin-deficient mice increased release of Cxcl2 but did not increase IL-1beta release. To define the mechanism of hyaluronan-mediated activation of cryopyrin, elements of the hyaluronan recognition process were studied in detail. IL-1beta release was inhibited in peritoneal macrophages derived from CD44-deficient mice, in an MH-S macrophage cell line treated with antibodies to CD44, or by inhibitors of lysosome function. The requirement for CD44 binding and hyaluronan internalization could be bypassed by intracellular administration of hyaluronan oligosaccharides (10-18-mer) in lipopolysaccharide-primed macrophages. Therefore, the action of CD44 and subsequent hyaluronan catabolism trigger the intracellular cryopyrin --> IL-1beta pathway. These findings support the hypothesis that hyaluronan works through IL-1beta and the cryopyrin system to signal sterile inflammation.
Figures



References
-
- Beutler, B. (2004) Nature 430 257–263 - PubMed
-
- Beg, A. A. (2002) Trends Immunol. 23 509–512 - PubMed
-
- Johnson, G. B., Brunn, G. J., Kodaira, Y., and Platt, J. L. (2002) J. Immunol. 168 5233–5239 - PubMed
-
- Tammi, R., Saamanen, A. M., Maibach, H. I., and Tammi, M. (1991) J. Investig. Dermatol. 97 126–130 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

