Cell polarity triggered by cell-cell adhesion via E-cadherin
- PMID: 19258396
- PMCID: PMC2720926
- DOI: 10.1242/jcs.028183
Cell polarity triggered by cell-cell adhesion via E-cadherin
Abstract
Cell polarity is orchestrated by numerous extracellular cues, and guides events such as chemotaxis, mitosis and wound healing. In scrape-wound assays of cell monolayers, wound-edge cells orient their centrosomes towards the wound, a process that appears to depend on the formation of new cell-extracellular-matrix adhesions as cells spread into the wound. In direct contrast to scrape-wounded cells, isolated cells without cell-cell contacts failed to polarize, suggesting that asymmetry of cell-cell adhesions resulting from monolayer disruption might contribute to polarization. By using micropatterned substrates to engineer such asymmetries in kidney epithelial cells, we found that cell-cell contact induced displacement of the nucleus towards the contact, and also caused centrosomal reorientation and lamellipodial ruffling to the distal side of the nucleus. Upon release from micropatterned constraints, cells exhibited directed migration away from the cell-cell contact. Disrupting E-cadherin engagement randomized nuclear position and lamellipodial ruffling in patterned cultures, and abrogated scrape-wound-induced cell reorientation, but not migration rate. Polarity that was induced by cell-cell contact required an intact actin cytoskeleton and Cdc42 activity, but not RhoA or Rac signaling. Together, these findings demonstrate a novel role for cell-cell adhesion in polarization, and have implications for wound healing and developmental patterning.
Figures

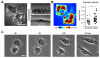

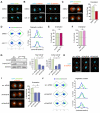
References
-
- Braga, V. M. and Yap, A. S. (2005). The challenges of abundance: epithelial junctions and small GTPase signalling. Curr. Opin. Cell Biol. 17, 466-474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

