U19/Eaf2 binds to and stabilizes von hippel-lindau protein
- PMID: 19258512
- PMCID: PMC2806597
- DOI: 10.1158/0008-5472.CAN-08-2595
U19/Eaf2 binds to and stabilizes von hippel-lindau protein
Abstract
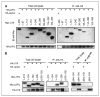
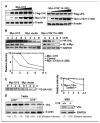
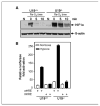
Studies have firmly established a key regulatory role for the tumor suppressor pVHL in the regulation of the vascular system and normal spermatogenesis. Here, we report that knockout of the newly identified tumor suppressor U19/Eaf2 also caused vascular system abnormalities and aspermatogenesis, suggesting a potential link between U19/Eaf2 and pVHL. Coimmunoprecipitation and in vitro binding assays showed an association between U19/Eaf2 and pVHL, whereas deletion mutagenesis revealed the requirement of the NH(2) terminus of U19/Eaf2 and both the alpha and beta domains of pVHL for this binding. U19/Eaf2 stabilizes pVHL, as shown by protein stability and pulse-chase studies. Testes and mouse embryonic fibroblasts (MEF) derived from U19/Eaf2 knockout mice expressed reduced levels of pVHL, indicating that full in vivo expression of pVHL indeed requires U19/Eaf2. As expected, U19/Eaf2 knockout MEF cells exhibited an increased level and activity of hypoxia-inducible factor 1alpha (HIF1alpha), a protein typically regulated via a pVHL-mediated degradation pathway. Furthermore, angiogenesis in a Matrigel plug assay was significantly increased in U19/Eaf2 knockout mice. The above observations argue that U19/Eaf2 can modulate HIF1alpha and angiogenesis, possibly via direct binding and stabilization of pVHL.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures



Similar articles
-
EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate.Angiogenesis. 2011 Sep;14(3):331-43. doi: 10.1007/s10456-011-9217-1. Epub 2011 Jun 3. Angiogenesis. 2011. PMID: 21638067 Free PMC article.
-
Tumor suppressor U19/EAF2 regulates thrombospondin-1 expression via p53.Oncogene. 2010 Jan 21;29(3):421-31. doi: 10.1038/onc.2009.326. Epub 2009 Oct 12. Oncogene. 2010. PMID: 19826414 Free PMC article.
-
Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein.J Biol Chem. 2000 Aug 18;275(33):25733-41. doi: 10.1074/jbc.M002740200. J Biol Chem. 2000. PMID: 10823831
-
Regulation of HIF by the von Hippel-Lindau tumour suppressor: implications for cellular oxygen sensing.IUBMB Life. 2001 Jul;52(1-2):43-7. doi: 10.1080/15216540252774757. IUBMB Life. 2001. PMID: 11795592 Review.
-
The von Hippel-Lindau tumor suppressor, hypoxia-inducible factor-1 (HIF-1) degradation, and cancer pathogenesis.Semin Cancer Biol. 2003 Feb;13(1):83-9. doi: 10.1016/s1044-579x(02)00103-7. Semin Cancer Biol. 2003. PMID: 12507560 Review.
Cited by
-
EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate.Angiogenesis. 2011 Sep;14(3):331-43. doi: 10.1007/s10456-011-9217-1. Epub 2011 Jun 3. Angiogenesis. 2011. PMID: 21638067 Free PMC article.
-
FOXC1-mediated LINC00301 facilitates tumor progression and triggers an immune-suppressing microenvironment in non-small cell lung cancer by regulating the HIF1α pathway.Genome Med. 2020 Sep 2;12(1):77. doi: 10.1186/s13073-020-00773-y. Genome Med. 2020. PMID: 32878637 Free PMC article.
-
EAF2 regulates DNA repair through Ku70/Ku80 in the prostate.Oncogene. 2017 Apr;36(15):2054-2065. doi: 10.1038/onc.2016.373. Epub 2016 Oct 10. Oncogene. 2017. PMID: 27721405 Free PMC article.
-
Regulation of fertility, survival, and cuticle collagen function by the Caenorhabditis elegans eaf-1 and ell-1 genes.J Biol Chem. 2011 Oct 14;286(41):35915-35921. doi: 10.1074/jbc.M111.270454. Epub 2011 Aug 31. J Biol Chem. 2011. PMID: 21880729 Free PMC article.
-
ELL Protein-associated Factor 2 (EAF2) Inhibits Transforming Growth Factor β Signaling through a Direct Interaction with Smad3.J Biol Chem. 2015 Oct 23;290(43):25933-45. doi: 10.1074/jbc.M115.663542. Epub 2015 Sep 14. J Biol Chem. 2015. PMID: 26370086 Free PMC article.
References
-
- Kaelin WG., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–82. - PubMed
-
- Linehan WM, Lerman MI, Zbar B. Identification of the von Hippel-Lindau (VHL) gene. Its role in renal cancer JAMA. 1995;273:564–70. - PubMed
-
- Linehan WM, Vasselli J, Srinivasan R, et al. Genetic basis of cancer of the kidney: disease-specific approaches to therapy. Clin Cancer Res. 2004;10:6282–9S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

