CYP2C9 amino acid residues influencing phenytoin turnover and metabolite regio- and stereochemistry
- PMID: 19258521
- PMCID: PMC2683772
- DOI: 10.1124/jpet.109.150706
CYP2C9 amino acid residues influencing phenytoin turnover and metabolite regio- and stereochemistry
Abstract
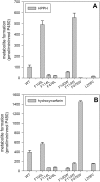
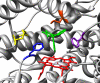
Phenytoin has been an effective anticonvulsant agent for over 60 years, although its clinical use is complicated by nonlinear pharmacokinetics, a narrow therapeutic index, and metabolically based drug-drug interactions. Although it is well established that CYP2C9 is the major cytochrome P450 enzyme controlling metabolic elimination of phenytoin through its oxidative conversion to (S)-5-(4-hydroxyphenyl)-5-phenylhydantoin (p-HPPH), nothing is known about the amino acid binding determinants within the CYP2C9 active site that promote metabolism and maintain the tight stereocontrol of hydroxy metabolite formation. This knowledge gap was addressed here through the construction of nine active site mutants at amino acid positions Phe100, Arg108, Phe114, Leu208, and Phe476 and in vitro analysis of the steady-state kinetics and stereochemistry of p-HPPH formation. The F100L and F114W mutants exhibited 4- to 5-fold increases in catalytic efficiency, whereas the F100W, F114L, F476L, and F476W mutants lost >90% of their phenytoin hydroxylation capacity. This pattern of effects differs substantially from that found previously for (S)-warfarin and (S)-flurbiprofen metabolism, suggesting that these three ligands bind within discrete locations in the CYP2C9 active site. Only the F114L, F476L, and L208V mutants altered phenytoin's orientation during catalytic turnover. The L208V mutant also uniquely demonstrated enhanced 6-hydroxylation of (S)-warfarin. These latter data provide the first experimental evidence for a role of the F-G loop region in dictating the catalytic orientation of substrates within the CYP2C9 active site.
Figures


Similar articles
-
Functional analysis of phenylalanine residues in the active site of cytochrome P450 2C9.Biochemistry. 2008 Nov 11;47(45):11725-34. doi: 10.1021/bi801231m. Epub 2008 Oct 16. Biochemistry. 2008. PMID: 18922023 Free PMC article.
-
Enzymatic determinants of the substrate specificity of CYP2C9: role of B'-C loop residues in providing the pi-stacking anchor site for warfarin binding.Biochemistry. 1999 Mar 16;38(11):3285-92. doi: 10.1021/bi982161+. Biochemistry. 1999. PMID: 10079071
-
Human CYP2C-mediated stereoselective phenytoin hydroxylation in Japanese: difference in chiral preference of CYP2C9 and CYP2C19.Biochem Pharmacol. 1999 Jun 1;57(11):1297-303. doi: 10.1016/s0006-2952(99)00034-9. Biochem Pharmacol. 1999. PMID: 10230773
-
Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development.Curr Med Chem. 2009;16(27):3480-675. doi: 10.2174/092986709789057635. Epub 2009 Sep 1. Curr Med Chem. 2009. PMID: 19515014 Review.
-
New insights into the structural features and functional relevance of human cytochrome P450 2C9. Part II.Curr Drug Metab. 2009 Dec;10(10):1127-50. doi: 10.2174/138920009790820101. Curr Drug Metab. 2009. PMID: 20167000 Review.
Cited by
-
Search for the molecular basis of ultra-rapid CYP2C9-catalysed metabolism: relationship between SNP IVS8-109A>T and the losartan metabolism phenotype in Swedes.Eur J Clin Pharmacol. 2012 Jul;68(7):1033-42. doi: 10.1007/s00228-012-1210-0. Epub 2012 Feb 1. Eur J Clin Pharmacol. 2012. PMID: 22294058
-
Blood-brain barrier P450 enzymes and multidrug transporters in drug resistance: a synergistic role in neurological diseases.Curr Drug Metab. 2011 Oct;12(8):742-9. doi: 10.2174/138920011798357051. Curr Drug Metab. 2011. PMID: 21568937 Free PMC article. Review.
-
In vitro metabolism of exemestane by hepatic cytochrome P450s: impact of nonsynonymous polymorphisms on formation of the active metabolite 17β-dihydroexemestane.Pharmacol Res Perspect. 2017 Apr 27;5(3):e00314. doi: 10.1002/prp2.314. eCollection 2017 Jun. Pharmacol Res Perspect. 2017. PMID: 28603633 Free PMC article.
-
Intramolecular heme ligation of the cytochrome P450 2C9 R108H mutant demonstrates pronounced conformational flexibility of the B-C loop region: implications for substrate binding.Biochemistry. 2010 Oct 12;49(40):8700-8. doi: 10.1021/bi100911q. Epub 2010 Sep 21. Biochemistry. 2010. PMID: 20815369 Free PMC article.
-
Structural characterization of human cytochrome P450 2C19: active site differences between P450s 2C8, 2C9, and 2C19.J Biol Chem. 2012 Dec 28;287(53):44581-91. doi: 10.1074/jbc.M112.424895. Epub 2012 Nov 1. J Biol Chem. 2012. PMID: 23118231 Free PMC article.
References
-
- Argikar UA, Cloyd JC, Birnbaum AK, Leppik IE, Conway J, Kshirsagar S, Oetting WS, Klein EC, and Remmel RP (2006) Paradoxical urinary phenytoin metabolite (S)/(R) ratios in CYP2C19*1/*2 patients. Epilepsy Res 71 54-63. - PubMed
-
- Bajpai M, Roskos LK, Shen DD, and Levy RH (1996) Roles of cytochrome P4502C9 and cytochrome P4502C19 in the stereoselective metabolism of phenytoin to its major metabolite. Drug Metab Dispos 24 1401-1403. - PubMed
-
- Bush ED, Low LK, and Trager WF (1983) A sensitive and specific stable isotope assay for warfarin and its metabolites. Biomed Mass Spectrom 10 395-398. - PubMed
-
- Cheesman MJ, Baer BR, Zheng YM, Gillam EM, and Rettie AE (2003) Rabbit CYP4B1 engineered for high-level expression in Escherichia coli: ligand stabilization and processing of the N-terminus and heme prosthetic group. Arch Biochem Biophys 416 17-24. - PubMed
-
- Dickmann LJ, Locuson CW, Jones JP, and Rettie AE (2004) Differential roles of Arg97, Asp293, and Arg108 in enzyme stability and substrate specificity of CYP2C9. Mol Pharmacol 65 842-850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

