BAFF-R promotes cell proliferation and survival through interaction with IKKbeta and NF-kappaB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells
- PMID: 19258594
- PMCID: PMC2680367
- DOI: 10.1182/blood-2008-10-183467
BAFF-R promotes cell proliferation and survival through interaction with IKKbeta and NF-kappaB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells
Abstract
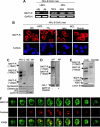
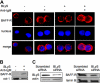
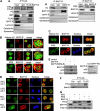
BLyS and its major receptor BAFF-R have been shown to be critical for development and homeostasis of normal B lymphocytes, and for cell growth and survival of neoplastic B lymphocytes, but the biologic mechanisms of this ligand/receptor-derived intracellular signaling pathway(s) have not been completely defined. We have discovered that the BAFF-R protein was present in the cell nucleus, in addition to its integral presence in the plasma membrane and cytoplasm, in both normal and neoplastic B cells. BAFF-R interacted with histone H3 and IKKbeta in the cell nucleus, enhancing histone H3 phosphorylation through IKKbeta. Nuclear BAFF-R was also associated with NF-kappaB/c-Rel and bound to NF-kappaB targeted promoters including BLyS, CD154, Bcl-xL, IL-8, and Bfl-1/A1, promoting the transcription of these genes. These observations suggested that in addition to activating NF-kappaB pathways in the plasma membrane, BAFF-R also promotes normal B-cell and B-cell non-Hodgkin lymphoma (NHL-B) survival and proliferation by functioning as a transcriptional regulator through a chromatin remodeling mechanism(s) and NF-kappaB association. Our studies provide an expanded conceptual view of the BAFF-R signaling, which should contribute a better understanding of the physiologic mechanisms involved in normal B-cell survival and growth, as well as in the pathophysiology of aggressive B-cell malignancies and autoimmune diseases.
Figures




Similar articles
-
Constitutive BR3 receptor signaling in diffuse, large B-cell lymphomas stabilizes nuclear factor-κB-inducing kinase while activating both canonical and alternative nuclear factor-κB pathways.Blood. 2011 Jan 6;117(1):200-10. doi: 10.1182/blood-2010-06-290437. Epub 2010 Oct 1. Blood. 2011. PMID: 20889926 Free PMC article.
-
Nuclear CD40 interacts with c-Rel and enhances proliferation in aggressive B-cell lymphoma.Blood. 2007 Sep 15;110(6):2121-7. doi: 10.1182/blood-2007-02-073080. Epub 2007 Jun 13. Blood. 2007. PMID: 17567982 Free PMC article.
-
Critical role of B cell lymphoma 10 in BAFF-regulated NF-κB activation and survival of anergic B cells.J Immunol. 2012 Dec 1;189(11):5185-93. doi: 10.4049/jimmunol.1102952. Epub 2012 Oct 19. J Immunol. 2012. PMID: 23087406 Free PMC article.
-
Roles of TRAFs in NF-κB signaling pathways mediated by BAFF.Immunol Lett. 2018 Apr;196:113-118. doi: 10.1016/j.imlet.2018.01.010. Epub 2018 Feb 20. Immunol Lett. 2018. PMID: 29378215 Review.
-
Rel/NF-kappaB transcription factors: key mediators of B-cell activation.Immunol Rev. 2000 Aug;176:134-40. doi: 10.1034/j.1600-065x.2000.00615.x. Immunol Rev. 2000. PMID: 11043773 Review.
Cited by
-
Constitutive BR3 receptor signaling in diffuse, large B-cell lymphomas stabilizes nuclear factor-κB-inducing kinase while activating both canonical and alternative nuclear factor-κB pathways.Blood. 2011 Jan 6;117(1):200-10. doi: 10.1182/blood-2010-06-290437. Epub 2010 Oct 1. Blood. 2011. PMID: 20889926 Free PMC article.
-
BAFF activates Erk1/2 promoting cell proliferation and survival by Ca2+-CaMKII-dependent inhibition of PP2A in normal and neoplastic B-lymphoid cells.Biochem Pharmacol. 2014 Jan 15;87(2):332-43. doi: 10.1016/j.bcp.2013.11.006. Epub 2013 Nov 22. Biochem Pharmacol. 2014. PMID: 24269630 Free PMC article.
-
Potential effects of CRM1 inhibition in mantle cell lymphoma.Chin J Cancer Res. 2012 Dec;24(4):374-87. doi: 10.3978/j.issn.1000-9604.2012.09.05. Chin J Cancer Res. 2012. PMID: 23357869 Free PMC article.
-
RNA-binding protein Ptbp1 is essential for BCR-mediated antibody production.Int Immunol. 2019 Mar 5;31(3):157-166. doi: 10.1093/intimm/dxy077. Int Immunol. 2019. PMID: 30476084 Free PMC article.
-
B cells in chronic graft-versus-host disease.Biol Blood Marrow Transplant. 2015 Jan;21(1):16-23. doi: 10.1016/j.bbmt.2014.10.029. Epub 2014 Nov 12. Biol Blood Marrow Transplant. 2015. PMID: 25452031 Free PMC article. Review.
References
-
- Yan M, Brady JR, Chan B, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. - PubMed
-
- He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol. 2004;172:3268–3279. - PubMed
-
- Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3:958–965. - PubMed
-
- Kayagaki N, Yan M, Seshasayee D, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;17:515–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

