Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement
- PMID: 19258595
- PMCID: PMC2686137
- DOI: 10.1182/blood-2008-09-181107
Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement
Abstract
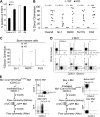
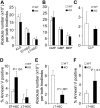
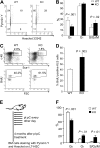
Currently, there is a major need in hematopoietic stem cell (HSC) transplantation to develop reduced-intensity regimens that do not cause DNA damage and associated toxicities and that allow a wider range of patients to receive therapy. Cytokine receptor signals through c-Kit and c-Mpl can modulate HSC quiescence and engraftment, but the intracellular signals and transcription factors that mediate these effects during transplantation have not been defined. Here we show that loss of one allele of signal transducer and activator of transcription 5 (STAT5) in nonablated adult mutant mice permitted engraftment with wild-type HSC. Conditional deletion of STAT5 using Mx1-Cre caused maximal reduction in STAT5 mRNA (> 97%) and rapidly decreased quiescence-associated c-Mpl downstream targets (Tie-2, p57), increased HSC cycling, and gradually reduced survival and depleted the long-term HSC pool. Host deletion of STAT5 was persistent and permitted efficient donor long-term HSC engraftment in primary and secondary hosts in the absence of ablative conditioning. Overall, these studies establish proof of principle for targeting of STAT5 as novel transplantation conditioning and demonstrate, for the first time, that STAT5, a mitogenic factor in most cell types, including hematopoietic progenitors, is a key transcriptional regulator that maintains quiescence of HSC during steady-state hematopoiesis.
Figures

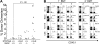

Similar articles
-
Gab2 promotes hematopoietic stem cell maintenance and self-renewal synergistically with STAT5.PLoS One. 2010 Feb 10;5(2):e9152. doi: 10.1371/journal.pone.0009152. PLoS One. 2010. PMID: 20161778 Free PMC article.
-
A STAT5 modifier locus on murine chromosome 7 modulates engraftment of hematopoietic stem cells during steady-state hematopoiesis.Blood. 2005 Feb 15;105(4):1476-83. doi: 10.1182/blood-2004-06-2302. Epub 2004 Oct 21. Blood. 2005. PMID: 15498858
-
Hematopoietic-repopulating defects from STAT5-deficient bone marrow are not fully accounted for by loss of thrombopoietin responsiveness.Blood. 2004 Apr 15;103(8):2965-72. doi: 10.1182/blood-2003-08-2963. Epub 2003 Dec 30. Blood. 2004. PMID: 15070672
-
STAT5 in hematopoietic stem cell biology and transplantation.JAKSTAT. 2013 Oct 1;2(4):e27159. doi: 10.4161/jkst.27159. Epub 2013 Nov 19. JAKSTAT. 2013. PMID: 24498540 Free PMC article. Review.
-
Rho GTPases and regulation of hematopoietic stem cell localization.Methods Enzymol. 2008;439:365-93. doi: 10.1016/S0076-6879(07)00427-2. Methods Enzymol. 2008. PMID: 18374178 Review.
Cited by
-
An intrinsic BM hematopoietic niche occupancy defect of HSC in scid mice facilitates exogenous HSC engraftment.Blood. 2012 Feb 16;119(7):1768-71. doi: 10.1182/blood-2011-05-350611. Epub 2011 Dec 6. Blood. 2012. PMID: 22147896 Free PMC article.
-
The Derlin-1-Stat5b axis maintains homeostasis of adult hippocampal neurogenesis.EMBO Rep. 2024 Aug;25(8):3678-3706. doi: 10.1038/s44319-024-00205-7. Epub 2024 Jul 30. EMBO Rep. 2024. PMID: 39080439 Free PMC article.
-
Stem cell competition: finding balance in the niche.Trends Cell Biol. 2013 Aug;23(8):357-64. doi: 10.1016/j.tcb.2013.03.001. Epub 2013 Apr 16. Trends Cell Biol. 2013. PMID: 23597843 Free PMC article. Review.
-
Gab2 promotes hematopoietic stem cell maintenance and self-renewal synergistically with STAT5.PLoS One. 2010 Feb 10;5(2):e9152. doi: 10.1371/journal.pone.0009152. PLoS One. 2010. PMID: 20161778 Free PMC article.
-
STAT5-regulated microRNA-193b controls haematopoietic stem and progenitor cell expansion by modulating cytokine receptor signalling.Nat Commun. 2015 Nov 25;6:8928. doi: 10.1038/ncomms9928. Nat Commun. 2015. PMID: 26603207 Free PMC article.
References
-
- Nijnik A, Woodbine L, Marchetti C, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. - PubMed
-
- Down JD, Ploemacher RE. Transient and permanent engraftment potential of murine hematopoietic stem cell subsets: differential effects of host conditioning with gamma radiation and cytotoxic drugs. Exp Hematol. 1993;21:913–921. - PubMed
-
- Micklem HS, Clarke CM, Evans EP, Ford CE. Fate of chromosome-marked mouse bone marrow cells tranfused into normal syngeneic recipients. Transplantation. 1968;6:299–302. - PubMed
-
- Nilsson SK, Dooner MS, Tiarks CY, Weier HU, Quesenberry PJ. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997;89:4013–4020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

