Fluorescence lifetime imaging of endogenous fluorophores in histopathology sections reveals differences between normal and tumor epithelium in carcinoma in situ of the breast
- PMID: 19259625
- PMCID: PMC5575754
- DOI: 10.1007/s12013-009-9046-7
Fluorescence lifetime imaging of endogenous fluorophores in histopathology sections reveals differences between normal and tumor epithelium in carcinoma in situ of the breast
Abstract
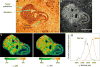
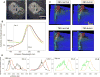
The classical examination of histology slides from a mouse model of breast cancer has been extended in this study to incorporate modern multiphoton excitation and photon-counting techniques. The advantage of such approaches is quantification of potential diagnostic parameters from the fluorescence emission signal, whereby the traditional descriptive staging process is complemented by measurements of fluorescence intensity, lifetime, and spectra. We explored whether the clinical "gold standard" of eosin and hematoxylin stained histology slides would provide optical biomarker signatures of diagnostic value. Alternatively, we examined unstained slides for changes in intensity and/or fluorescence lifetime of relevant endogenous fluorophores. Although eosin provided a strong emission signal and had distinct spectra and lifetime, we found that it was not useful as a fluorescent biological marker, particularly when combined with hematoxylin. Instead, we found that the properties of the fluorescence from the endogenous fluorophores NADH and FAD were indicative of the pathological state of the tissue. Comparing regions of carcinoma in situ to adjacent histologically normal regions, we found that tumor cells produced higher intensity and had a longer fluorescence lifetime. By imaging at 780 nm and 890 nm excitation, we were able to differentiate the fluorescence of FAD from NADH by separating the emission spectra. The shift to a longer lifetime in tumor cells was independent of the free or bound state of FAD and NADH, and of the excitation wavelength. Most forms of cancer have altered metabolism and redox ratios; here we present a method that has potential for early detection of these changes, which are preserved in fixed tissue samples such as classic histopathology slides.
Figures



References
-
- Eliceiri KW, Fan CH, Lyons GE, White JG. Analysis of histology specimens using lifetime multiphoton microscopy. Journal of Biomedical Optics. 2003;8:376–380. - PubMed
-
- Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation–reduction states in vivo. Science. 1962;137:499–508. - PubMed
-
- Warburg O, Dickens F, Kaiser Wilhelm-Institut für Biologie, B . The metabolism of tumours: Investigations from the Kaiser-Wilhelm Institute for Biology. Berlin-Dahlem. London: Constable; 1930.
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer. 2004;4:891–899. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

