Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1
- PMID: 19260765
- PMCID: PMC2650726
- DOI: 10.1371/journal.pbio.1000060
Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1
Abstract
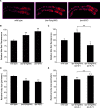
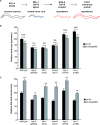
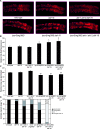
The target of rapamycin (TOR) kinase coordinately regulates fundamental metabolic and cellular processes to support growth, proliferation, survival, and differentiation, and consequently it has been proposed as a therapeutic target for the treatment of cancer, metabolic disease, and aging. The TOR kinase is found in two biochemically and functionally distinct complexes, termed TORC1 and TORC2. Aided by the compound rapamycin, which specifically inhibits TORC1, the role of TORC1 in regulating translation and cellular growth has been extensively studied. The physiological roles of TORC2 have remained largely elusive due to the lack of pharmacological inhibitors and its genetic lethality in mammals. Among potential targets of TORC2, the pro-survival kinase AKT has garnered much attention. Within the context of intact animals, however, the physiological consequences of phosphorylation of AKT by TORC2 remain poorly understood. Here we describe viable loss-of-function mutants in the Caenorhabditis elegans homolog of the TORC2-specific component, Rictor (CeRictor). These mutants display a mild developmental delay and decreased body size, but have increased lipid storage. These functions of CeRictor are not mediated through the regulation of AKT kinases or their major downstream target, the insulin-regulated FOXO transcription factor DAF-16. We found that loss of sgk-1, a homolog of the serum- and glucocorticoid-induced kinase, mimics the developmental, growth, and metabolic phenotypes of CeRictor mutants, while a novel, gain-of-function mutation in sgk-1 suppresses these phenotypes, indicating that SGK-1 is a mediator of CeRictor activity. These findings identify new physiological roles for TORC2, mediated by SGK, in regulation of C. elegans lipid accumulation and growth, and they challenge the notion that AKT is the primary effector of TORC2 function.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
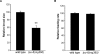
Similar articles
-
Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans.Genes Dev. 2009 Feb 15;23(4):496-511. doi: 10.1101/gad.1775409. Genes Dev. 2009. PMID: 19240135 Free PMC article.
-
A non-canonical role for the C. elegans dosage compensation complex in growth and metabolic regulation downstream of TOR complex 2.Development. 2013 Sep;140(17):3601-12. doi: 10.1242/dev.094292. Epub 2013 Jul 24. Development. 2013. PMID: 23884442 Free PMC article.
-
Effects of Caenorhabditis elegans sgk-1 mutations on lifespan, stress resistance, and DAF-16/FoxO regulation.Aging Cell. 2013 Oct;12(5):932-40. doi: 10.1111/acel.12120. Epub 2013 Jul 19. Aging Cell. 2013. PMID: 23786484 Free PMC article.
-
TOR Signaling in Caenorhabditis elegans Development, Metabolism, and Aging.Genetics. 2019 Oct;213(2):329-360. doi: 10.1534/genetics.119.302504. Genetics. 2019. PMID: 31594908 Free PMC article. Review.
-
DAF-16: FOXO in the Context of C. elegans.Curr Top Dev Biol. 2018;127:1-21. doi: 10.1016/bs.ctdb.2017.11.007. Epub 2018 Feb 2. Curr Top Dev Biol. 2018. PMID: 29433733 Review.
Cited by
-
Reconstruction of signaling networks regulating fungal morphogenesis by transcriptomics.Eukaryot Cell. 2009 Nov;8(11):1677-91. doi: 10.1128/EC.00050-09. Epub 2009 Sep 11. Eukaryot Cell. 2009. PMID: 19749177 Free PMC article.
-
TOR and ageing: a complex pathway for a complex process.Philos Trans R Soc Lond B Biol Sci. 2011 Jan 12;366(1561):17-27. doi: 10.1098/rstb.2010.0198. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21115526 Free PMC article. Review.
-
Evolution of the TOR pathway.J Mol Evol. 2011 Oct;73(3-4):209-20. doi: 10.1007/s00239-011-9469-9. Epub 2011 Nov 5. J Mol Evol. 2011. PMID: 22057117 Free PMC article.
-
mTORC2-SGK-1 acts in two environmentally responsive pathways with opposing effects on longevity.Aging Cell. 2014 Oct;13(5):869-78. doi: 10.1111/acel.12248. Epub 2014 Jul 9. Aging Cell. 2014. PMID: 25040785 Free PMC article.
-
Mitochondrial-Nuclear Epistasis Impacts Fitness and Mitochondrial Physiology of Interpopulation Caenorhabditis briggsae Hybrids.G3 (Bethesda). 2015 Nov 19;6(1):209-19. doi: 10.1534/g3.115.022970. G3 (Bethesda). 2015. PMID: 26585825 Free PMC article.
References
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. - PubMed
-
- Tsang CK, Qi H, Liu LF, Zheng XF. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today. 2007;12:112–124. - PubMed
-
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. - PubMed
-
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. - PubMed
-
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

