The interaction of Bacteroides fragilis with components of the human fibrinolytic system
- PMID: 19260960
- PMCID: PMC2719845
- DOI: 10.1111/j.1574-695X.2009.00546.x
The interaction of Bacteroides fragilis with components of the human fibrinolytic system
Abstract
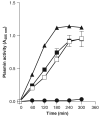
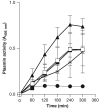
Bacteroides fragilis is a minor component of the intestinal microbiota and the most frequently isolated from intra-abdominal infections and bacteremia. Previously, our group has shown that molecules involved in laminin-1 (LMN-1) recognition were present in outer membrane protein extracts of B. fragilis MC2 strain. One of these proteins was identified and showed 98% similarity to a putative B. fragilis plasminogen-binding protein precursor, deposited in the public database. Thus, the objective of this work was to overexpress and further characterize this novel adhesin. The ability of B. fragilis MC2 strain and purified protein to convert plasminogen into plasmin was tested. Our results showed that B. fragilis strain MC2 strain adhered to both LMN-1 and plasminogen and this adhesion was inhibited by either LMN-1 or plasminogen. Regarding the plasminogen activation activity, both the whole bacterial cell and the purified protein converted plasminogen into plasmin similar to streptokinase used as a positive control. Bacterial receptors that recognize plasminogen bind to it and enhance its activation, transforming a nonproteolytic bacterium into a proteolytic one. We present in vitro evidence for a pathogenic function of the plasminogen receptor in promoting adherence to laminin and also the formation of plasmin by B. fragilis.
Figures
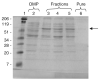
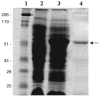

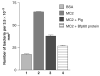
Similar articles
-
The Bfp60 surface adhesin is an extracellular matrix and plasminogen protein interacting in Bacteroides fragilis.Int J Med Microbiol. 2013 Dec;303(8):492-7. doi: 10.1016/j.ijmm.2013.06.007. Epub 2013 Jun 21. Int J Med Microbiol. 2013. PMID: 23850366 Free PMC article.
-
A Bacteroides fragilis surface glycoprotein mediates the interaction between the bacterium and the extracellular matrix component laminin-1.Res Microbiol. 2006 Dec;157(10):960-6. doi: 10.1016/j.resmic.2006.09.005. Epub 2006 Oct 26. Res Microbiol. 2006. PMID: 17125972
-
Pbp, a cell-surface exposed plasminogen binding protein of Bacteroides fragilis.Microbes Infect. 2008 Apr;10(5):514-21. doi: 10.1016/j.micinf.2008.01.015. Epub 2008 Feb 8. Microbes Infect. 2008. PMID: 18403231
-
The Bacteroides fragilis cell envelope: quarterback, linebacker, coach-or all three?Anaerobe. 2006 Oct-Dec;12(5-6):211-20. doi: 10.1016/j.anaerobe.2006.09.004. Epub 2006 Oct 10. Anaerobe. 2006. PMID: 17045496 Review.
-
Molecular mechanisms of plasminogen activation: bacterial cofactors provide clues.Trends Biochem Sci. 2000 Feb;25(2):53-9. doi: 10.1016/s0968-0004(99)01521-2. Trends Biochem Sci. 2000. PMID: 10664583 Review.
Cited by
-
Bacterial plasminogen receptors: mediators of a multifaceted relationship.J Biomed Biotechnol. 2012;2012:272148. doi: 10.1155/2012/272148. Epub 2012 Oct 14. J Biomed Biotechnol. 2012. PMID: 23118502 Free PMC article. Review.
-
Interaction of Bacteroides fragilis and Bacteroides thetaiotaomicron with the kallikrein-kinin system.Microbiology (Reading). 2011 Jul;157(Pt 7):2094-2105. doi: 10.1099/mic.0.046862-0. Epub 2011 Apr 28. Microbiology (Reading). 2011. PMID: 21527472 Free PMC article.
-
The Bfp60 surface adhesin is an extracellular matrix and plasminogen protein interacting in Bacteroides fragilis.Int J Med Microbiol. 2013 Dec;303(8):492-7. doi: 10.1016/j.ijmm.2013.06.007. Epub 2013 Jun 21. Int J Med Microbiol. 2013. PMID: 23850366 Free PMC article.
-
Binding of the extracellular matrix laminin-1 to Clostridioides difficile strains.Mem Inst Oswaldo Cruz. 2022 Jun 17;117:e220035. doi: 10.1590/0074-02760220035. eCollection 2022. Mem Inst Oswaldo Cruz. 2022. PMID: 35730804 Free PMC article.
-
Identification of the alpha-enolase P46 in the extracellular membrane vesicles of Bacteroides fragilis.Mem Inst Oswaldo Cruz. 2018 Mar;113(3):178-184. doi: 10.1590/0074-02760170340. Mem Inst Oswaldo Cruz. 2018. PMID: 29412357 Free PMC article.
References
-
- Blatch GL, Lässle M. The tetratricopeptide repeat: a structutral motif mediating protein–protein interaction. BioEssays. 1999;21:932–939. - PubMed
-
- Boyle MD, Lottenberg R. Plasminogen activation by invasion human pathogens. Thromb Haemostasis. 1997;77:1–10. - PubMed
-
- Brook I, Frasier EH. Aerobic and anaerobic microbiology in intra-abdominal infections associated with diverticulitis. J Med Microbiol. 2000;49:827–830. - PubMed
-
- Dabo SM, Confer AW, Saliki JT, Anderson BE. Binding of Bordetella henselae to extracelullar molecules: identification of potential adhesins. Microb Pathogenesis. 2006;41:10–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
