High frequency of corticosteroid and immunosuppressive therapy in patients with systemic sclerosis despite limited evidence for efficacy
- PMID: 19261182
- PMCID: PMC2688174
- DOI: 10.1186/ar2634
High frequency of corticosteroid and immunosuppressive therapy in patients with systemic sclerosis despite limited evidence for efficacy
Abstract
Introduction: In systemic sclerosis (SSc) little evidence for the effectiveness of anti-inflammatory and immunosuppressive therapy exists. The objective of this study was to determine the extent to which SSc patients are treated with corticosteroids and immunosuppressive agents.
Methods: Data on duration and dosage of corticosteroids and on the type of immunosuppressive agent were analyzed from 1,729 patients who were registered in the German Network for Systemic Scleroderma (DNSS).
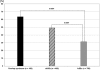
Results: A total 41.3% of all registered SSc patients was treated with corticosteroids. Corticosteroid use was reported in 49.1% of patients with diffuse cutaneous SSc and 31.3% of patients with limited cutaneous SSc (P < 0.0001). Among patients with overlap disease characteristics, 63.5% received corticosteroids (P < 0.0001 vs. limited cutaneous SSc). A total 16.1% of the patients received corticosteroids with a daily dose >or= 15 mg prednisone equivalent. Immunosuppressive therapy was prescribed in 35.8% of patients. Again, among those patients with overlap symptoms, a much higher proportion (64.1%) was treated with immunosuppressive agents, compared with 46.4% of those with diffuse cutaneous SSc sclerosis and 22.2% of those with limited cutaneous SSc (P < 0.0001). The most commonly prescribed drugs were methotrexate (30.5%), cyclophosphamide (22.2%), azathioprine (21.8%) and (hydroxy)chloroquine (7.2%). The use of these compounds varied significantly between medical subspecialties.
Conclusions: Despite limited evidence for the effectiveness of corticosteroids and immunosuppressive agents in SSc, these potentially harmful drugs are frequently prescribed to patients with all forms of SSc. Therefore, this study indicates the need to develop and communicate adequate treatment recommendations.
Figures

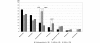
References
-
- Denton CP, Black CM. Scleroderma – clinical and pathological advances. Best Pract Res Clin Rheumatol. 2004;18:271–290. - PubMed
-
- White B, Bauer EA, Goldsmith LA. Guidelines for clinical trials in systemic sclerosis (scleroderma). I. Disease modifying interventions. The American College of Rheumatology Committee on Design and Outcomes in Clinical Trials in Systemic sclerosis. Arthritis Rheum. 1995;38:351–360. doi: 10.1002/art.1780380309. - DOI - PubMed
-
- White B, Moore WC, Wigley FM, Xiao HQ, Wise RA. Cyclophosphamide is associated with pulmonary function and survival benefit in patients with scleroderma and alveolitis. Ann Intern Med. 2000;132:947–954. - PubMed
-
- Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C, Bolster M, Seibold JR, Riley DJ, Hsu VM, Varga J, Schraufnagel DE, Theodore A, Simms R, Wise R, Wigley F, White B, Steen V, Read C, Mayes M, Parsley E, Mubarak K, Connolly MK, Golden J, Olman M, Fessler B, Rothfield N, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

