Ligand-independent thrombopoietin mutant receptor requires cell surface localization for endogenous activity
- PMID: 19261614
- PMCID: PMC2673247
- DOI: 10.1074/jbc.M808703200
Ligand-independent thrombopoietin mutant receptor requires cell surface localization for endogenous activity
Abstract
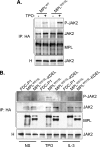
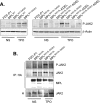
The activating W515L mutation in the thrombopoietin receptor (MPL) has been identified in primary myelofibrosis and essential thrombocythemia. MPL belongs to a subset of the cytokine receptor superfamily that requires the JAK2 kinase for signaling. We examined whether the ligand-independent MPL(W515L) mutant could signal intracellularly. Addition of the endoplasmic reticulum (ER) retention KDEL sequence to the receptor C terminus efficiently locked MPL(W515L) within its natural ER/Golgi maturation pathway. In contrast to cells expressing the parental MPL(W515L), MPL(W515L)-KDEL-expressing FDC-P1 cells were unable to grow autonomously and to produce tumors in nude mice. When observed, tumor nodules resulted from in vivo selection of cells leaking the receptor at their surface. JAK2 co-immunoprecipitated with MPL(W515L)-KDEL but was not phosphorylated. We generated disulfide-bonded MPL(W515L) homodimers by the S402C substitution, both in the normal and KDEL context. Unlike MPL(W515L)-KDEL, MPL(W515L-S402C)-KDEL signaled constitutively and exhibited cell surface localization. These data establish that MPL(W515L) with appended JAK2 matures through the ER/Golgi system in an inactive conformation and suggest that the MPL(W515L)/JAK2 complex requires membrane localization for JAK2 phosphorylation, resulting in autonomous receptor signaling.
Figures







Similar articles
-
MPL W515L mutation in pediatric essential thrombocythemia.Pediatr Blood Cancer. 2013 Aug;60(8):E52-4. doi: 10.1002/pbc.24500. Epub 2013 Feb 25. Pediatr Blood Cancer. 2013. PMID: 23441089
-
JAK2 V617F and MPL W515L/K mutations in Korean patients with essential thrombocythemia.Korean J Lab Med. 2010 Oct;30(5):474-6. doi: 10.3343/kjlm.2010.30.5.474. Korean J Lab Med. 2010. PMID: 20890078
-
JAK2(V617F) allele burden discriminates essential thrombocythemia from a subset of prefibrotic-stage primary myelofibrosis.Exp Hematol. 2009 Oct;37(10):1186-1193.e7. doi: 10.1016/j.exphem.2009.07.005. Epub 2009 Jul 17. Exp Hematol. 2009. PMID: 19616600
-
Thrombocytosis.Hematology Am Soc Hematol Educ Program. 2009:159-67. doi: 10.1182/asheducation-2009.1.159. Hematology Am Soc Hematol Educ Program. 2009. PMID: 20008195 Review.
-
Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms.Blood. 2017 Feb 9;129(6):667-679. doi: 10.1182/blood-2016-10-695940. Epub 2016 Dec 27. Blood. 2017. PMID: 28028029 Review.
Cited by
-
Thrombopoietin is required for full phenotype expression in a JAK2V617F transgenic mouse model of polycythemia vera.PLoS One. 2020 Jun 1;15(6):e0232801. doi: 10.1371/journal.pone.0232801. eCollection 2020. PLoS One. 2020. PMID: 32479500 Free PMC article.
-
Autophagosome-lysosome mediated secretion of the thrombopoietin receptor is modulated by distinct driver mutations of myeloproliferative neoplasm.Leukemia. 2025 Sep;39(9):2181-2195. doi: 10.1038/s41375-025-02676-6. Epub 2025 Jul 3. Leukemia. 2025. PMID: 40610764 Free PMC article.
-
The Thrombopoietin Receptor, MPL, Is a Therapeutic Target of Opportunity in the MPN.Front Oncol. 2021 Mar 10;11:641613. doi: 10.3389/fonc.2021.641613. eCollection 2021. Front Oncol. 2021. PMID: 33777803 Free PMC article. Review.
-
His499 Regulates Dimerization and Prevents Oncogenic Activation by Asparagine Mutations of the Human Thrombopoietin Receptor.J Biol Chem. 2016 Feb 5;291(6):2974-87. doi: 10.1074/jbc.M115.696534. Epub 2015 Dec 1. J Biol Chem. 2016. PMID: 26627830 Free PMC article.
-
Eliminative signaling by Janus kinases: role in the downregulation of associated receptors.J Cell Biochem. 2014 Jan;115(1):8-16. doi: 10.1002/jcb.24647. J Cell Biochem. 2014. PMID: 23959845 Free PMC article. Review.
References
-
- Broudy, V. C., and Kaushansky, K. (1995) J. Leukocyte Biol. 57 719–725 - PubMed
-
- Gurney, A. L., Carver-Moore, K., de Sauvage, F. J., and Moore, M. W. (1994) Science 265 1445–1447 - PubMed
-
- Kaushansky, K., Lok, S., Holly, R. D., Broudy, V. C., Lin, N., Bailey, M. C., Forstrom, J. W., Buddle, M. M., Oort, P. J., Hagen, F. S., Roth, G. J., Papayannopoulou, T., and Foster, D. C. (1994) Nature 369 568–571 - PubMed
-
- Vainchenker, W., Methia, N., Debili, N., Titeux, M., and Wendling, F. (1995) Thromb. Haemostasis 74 526–528 - PubMed
-
- Wendling, F. (1999) Haematologica 84 158–166 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

