Calcitonin, a regulator of the 25-hydroxyvitamin D3 1alpha-hydroxylase gene
- PMID: 19261615
- PMCID: PMC2670111
- DOI: 10.1074/jbc.M806561200
Calcitonin, a regulator of the 25-hydroxyvitamin D3 1alpha-hydroxylase gene
Abstract
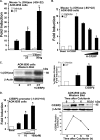
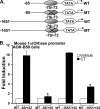
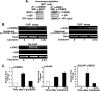
Although parathyroid hormone (PTH) induces 25-hydroxyvitamin D(3) (25(OH)D(3)) 1alpha-hydroxylase (1alpha(OH)ase) under hypocalcemic conditions, previous studies showed that calcitonin, not PTH, has an important role in the maintenance of serum 1,25-dihydroxyvitamin D(3) (1,25(OH)(2)D(3)) under normocalcemic conditions. In this study we report that 1alpha(OH)ase transcription is strongly induced by calcitonin in kidney cells and indicate mechanisms that underlie this regulation. The transcription factor C/EBPbeta is up-regulated by calcitonin in kidney cells and results in a significant enhancement of calcitonin induction of 1alpha(OH)ase transcription and protein expression. Mutation constructs of the 1alpha(OH)ase promoter demonstrate the importance of the C/EBPbeta binding site at -79/-73 for activation of the 1alpha(OH)ase promoter by calcitonin. The SWI/SNF chromatin remodeling complex was found to cooperate with calcitonin in the regulation of 1alpha(OH)ase. Chromatin immunoprecipitation analysis showed that calcitonin recruits C/EBPbeta to the 1alpha(OH)ase promoter, and Re-chromatin immunoprecipitation analysis (sequential chromatin immunoprecipitations using different antibodies) showed that C/EBPbeta and BRG1, an ATPase that is a component of the SWI/SNF complex, bind simultaneously to the 1alpha(OH)ase promoter. These findings are the first to address the dynamics between calcitonin, C/EBPbeta, and SWI/SNF in the regulation of 1alpha(OH)ase and provide a mechanism, for the first time, for calcitonin induction of 1alpha(OH)ase. Because plasma calcitonin as well as 1,25(OH)(2)D(3) have been reported to be increased during pregnancy and lactation and in early development, these findings suggest a mechanism that may account, at least in part, for the increase in plasma 1,25(OH)(2)D(3) during these times of increased calcium requirement.
Figures

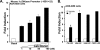
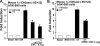

Similar articles
-
Functional cooperation between CCAAT/enhancer-binding proteins and the vitamin D receptor in regulation of 25-hydroxyvitamin D3 24-hydroxylase.Mol Cell Biol. 2005 Jan;25(1):472-87. doi: 10.1128/MCB.25.1.472-487.2005. Mol Cell Biol. 2005. PMID: 15601867 Free PMC article.
-
Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1alpha-hydroxylase gene in normocalcemic rats.Proc Natl Acad Sci U S A. 1999 Jul 6;96(14):8253-8. doi: 10.1073/pnas.96.14.8253. Proc Natl Acad Sci U S A. 1999. PMID: 10393981 Free PMC article.
-
25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis.Science. 1997 Sep 19;277(5333):1827-30. doi: 10.1126/science.277.5333.1827. Science. 1997. PMID: 9295274
-
The vitamin D3 1alpha-hydroxylase gene and its regulation by active vitamin D3.Biosci Biotechnol Biochem. 2011;75(2):208-13. doi: 10.1271/bbb.100684. Epub 2011 Feb 7. Biosci Biotechnol Biochem. 2011. PMID: 21307571 Review.
-
Prostatic 25-hydroxyvitamin D-1alpha-hydroxylase and its implication in prostate cancer.J Cell Biochem. 2003 Feb 1;88(2):315-22. doi: 10.1002/jcb.10342. J Cell Biochem. 2003. PMID: 12520532 Review.
Cited by
-
The vitamin d receptor in thyroid development and function.Eur Thyroid J. 2012 Oct;1(3):168-75. doi: 10.1159/000342363. Epub 2012 Sep 22. Eur Thyroid J. 2012. PMID: 24783016 Free PMC article.
-
Calcium-Sensing Receptors Control CYP27B1-Luciferase Expression: Transcriptional and Posttranscriptional Mechanisms.J Endocr Soc. 2021 Jun 5;5(9):bvab057. doi: 10.1210/jendso/bvab057. eCollection 2021 Sep 1. J Endocr Soc. 2021. PMID: 34337274 Free PMC article.
-
The Effect of Clothing on Vitamin D Status, Bone Turnover Markers, and Bone Mineral Density in Young Kuwaiti Females.Int J Endocrinol. 2019 Jun 23;2019:6794837. doi: 10.1155/2019/6794837. eCollection 2019. Int J Endocrinol. 2019. PMID: 31341474 Free PMC article.
-
The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer.Respir Res. 2011 Mar 18;12(1):31. doi: 10.1186/1465-9921-12-31. Respir Res. 2011. PMID: 21418564 Free PMC article. Review.
-
Does environmental confounding mask pleiotropic effects of a multiple sclerosis susceptibility variant on vitamin D in psychosis?NPJ Schizophr. 2015 Oct 28;1:15036. doi: 10.1038/npjschz.2015.36. eCollection 2015. NPJ Schizophr. 2015. PMID: 27336042 Free PMC article.
References
-
- Bikle, D., Adams, J., and Christakos, S. (2008) in Primer on Metabolic Bone Diseases and Disorder of Mineral Metabolism (Rosen, C., ed) pp. 141–149, American Society for Bone and Mineral Research, Washington DC
-
- Omdahl, J. L., Bobrovnikova, E. V., Annalora, A., Chen, P., and Serda, R. (2003) J. Cell. Biochem. 88, 356–362 - PubMed
-
- Henry, H. (2005) in Vitamin D (Feldman, D., Glorieux, F. H., and Pike, J. W., ed) pp. 69–83, Academic Press, Inc., San Diego, CA
-
- St-Arnaud, R., Messerlian, S., Moir, J. M., Omdahl, J. L., and Glorieux, F. H. (1997) J. Bone Miner. Res. 12, 1552–1559 - PubMed
-
- Kitanaka, S., Takeyama, K., Murayama, A., Sato, T., Okumura, K., Nogami, M., Hasegawa, Y., Niimi, H., Yanagisawa, J., Tanaka, T., and Kato, S. (1998) N. Engl. J. Med. 338, 653–661 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

