Comparative pathology of murine mucolipidosis types II and IIIC
- PMID: 19261645
- PMCID: PMC2705191
- DOI: 10.1354/vp.46-2-313
Comparative pathology of murine mucolipidosis types II and IIIC
Abstract
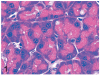
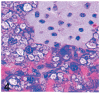
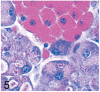
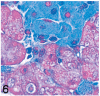
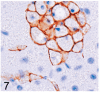
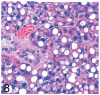
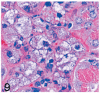
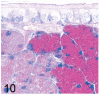
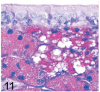
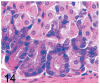
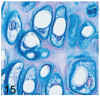
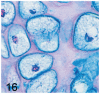
UDP-GlcNAc: lysosomal enzyme N-acetylglucosamine-1-phosphotransferase (GlcNAc-1-phosphotransferase) is an alpha(2)beta(2)gamma(2) hexameric enzyme that catalyzes the first step in the synthesis of the mannose 6-phosphate targeting signal on lysosomal hydrolases. In humans, mutations in the gene encoding the alpha/beta subunit precursor give rise to mucolipidosis II (MLII), whereas mutations in the gene encoding the gamma subunit cause the less severe mucolipidosis IIIC (MLIIIC). In this study we describe the phenotypic, histologic, and serum lysosomal enzyme abnormalities in knockout mice lacking the gamma subunit and compare these findings to those of mice lacking the alpha/beta subunits and humans with MLII and MLIIIC. We found that both lines of mutant mice had elevated levels of serum lysosomal enzymes and cytoplasmic alterations in secretory cells of several exocrine glands; however, lesions in gamma-subunit deficient (Gnptg(-/-)) mice were milder and more restricted in distribution than in alpha/beta-subunit deficient (Gnptab(-/-)) mice. We found that onset, extent, and severity of lesions that developed in these two different knockouts correlated with measured lysosomal enzyme activity; with a more rapid, widespread, and severe storage disease phenotype developing in Gnptab(-/-) mice. In contrast to mice deficient in the alpha/beta subunits, the mice lacking the gamma subunits were of normal size, lacked cartilage defects, and did not develop retinal degeneration. The milder disease in the gamma-subunit deficient mice correlated with residual synthesis of the mannose 6-phosphate recognition marker. Of significance, neither strain of mutant mice developed cytoplasmic vacuolar inclusions in fibrocytes or mesenchymal cells (I-cells), the characteristic lesion associated with the prominent skeletal and connective tissue abnormalities in humans with MLII and MLIII. Instead, the predominant lesions in both lines of mice were found in the secretory epithelial cells of several exocrine glands, including the pancreas, and the parotid, submandibular salivary, nasal, lacrimal, bulbourethral, and gastric glands. The absence of retinal and chondrocyte lesions in Gnptg(-/-) mice might be attributed to residual beta-glucuronidase activity. We conclude that mice lacking either alpha/beta or gamma subunits displayed clinical and pathologic features that differed substantially from those reported in humans having mutations in orthologous genes.
Figures





Similar articles
-
The lysosomal storage disorders mucolipidosis type II, type III alpha/beta, and type III gamma: Update on GNPTAB and GNPTG mutations.Hum Mutat. 2019 Jul;40(7):842-864. doi: 10.1002/humu.23748. Epub 2019 Apr 13. Hum Mutat. 2019. PMID: 30882951
-
Analysis of mucolipidosis II/III GNPTAB missense mutations identifies domains of UDP-GlcNAc:lysosomal enzyme GlcNAc-1-phosphotransferase involved in catalytic function and lysosomal enzyme recognition.J Biol Chem. 2015 Jan 30;290(5):3045-56. doi: 10.1074/jbc.M114.612507. Epub 2014 Dec 11. J Biol Chem. 2015. PMID: 25505245 Free PMC article.
-
Mucolipidosis II (I-cell disease) and mucolipidosis IIIA (classical pseudo-hurler polydystrophy) are caused by mutations in the GlcNAc-phosphotransferase alpha / beta -subunits precursor gene.Am J Hum Genet. 2006 Mar;78(3):451-63. doi: 10.1086/500849. Epub 2006 Jan 24. Am J Hum Genet. 2006. PMID: 16465621 Free PMC article.
-
Molecular analysis of the GlcNac-1-phosphotransferase.J Inherit Metab Dis. 2008 Apr;31(2):253-7. doi: 10.1007/s10545-008-0862-5. Epub 2008 Apr 15. J Inherit Metab Dis. 2008. PMID: 18425436 Review.
-
Mannose phosphorylation in health and disease.Eur J Cell Biol. 2010 Jan;89(1):117-23. doi: 10.1016/j.ejcb.2009.10.008. Epub 2009 Nov 28. Eur J Cell Biol. 2010. PMID: 19945768 Review.
Cited by
-
A role for inherited metabolic deficits in persistent developmental stuttering.Mol Genet Metab. 2012 Nov;107(3):276-80. doi: 10.1016/j.ymgme.2012.07.020. Epub 2012 Jul 28. Mol Genet Metab. 2012. PMID: 22884963 Free PMC article. Review.
-
High-throughput screening of mouse gene knockouts identifies established and novel skeletal phenotypes.Bone Res. 2014 Oct 28;2:14034. doi: 10.1038/boneres.2014.34. eCollection 2014. Bone Res. 2014. PMID: 26273529 Free PMC article.
-
Upregulation of Sortilin, a Lysosomal Sorting Receptor, Corresponds with Reduced Bioavailability of Latent TGFβ in Mucolipidosis II Cells.Biomolecules. 2020 Apr 26;10(5):670. doi: 10.3390/biom10050670. Biomolecules. 2020. PMID: 32357547 Free PMC article.
-
A GNPTAB nonsense variant is associated with feline mucolipidosis II (I-cell disease).BMC Vet Res. 2018 Dec 27;14(1):416. doi: 10.1186/s12917-018-1728-1. BMC Vet Res. 2018. PMID: 30591066 Free PMC article.
-
Histopathology reveals correlative and unique phenotypes in a high-throughput mouse phenotyping screen.Dis Model Mech. 2014 May;7(5):515-24. doi: 10.1242/dmm.015263. Epub 2014 Mar 20. Dis Model Mech. 2014. PMID: 24652767 Free PMC article.
References
-
- Adler AJ. Selective presence of acid hydrolases in the interphotoreceptor matrix. Exp Eye Res. 1989;49:1067–1077. - PubMed
-
- Bao M, Elmendorf BJ, Booth JL, Drake RR, Canfield WM. Bovine UDP-N-acetylglucosamine:-lysosomal-enzyme N-acetylglucosamine-1-phosphotransferase. II. Enzymatic characterization and identification of the catalytic subunit. J Biol Chem. 1996;271:31446–31451. - PubMed
-
- Bargal R, Zeigler M, Abu-Libdeh B, Zuri V, Mandel H, Ben Neriah Z, Stewart F, Elcioglu N, Hindi T, Le Merrer M, Bach G, Raas-Rothschild A. When mucolipidosis III meets mucolipidosis II: GNPTA gene mutations in 24 patients. Mol Genet Metab. 2006;88:359–363. - PubMed
-
- Beltrandelrio H, Kern F, Lanthorn T, Oravecz T, Piggott J, Powell D, Ramirez-Solis R, Sands AT, Zambrowicz BP. Saturation screening of the drug-gable mammalian genome. In: Carroll PM, Fitzgerald K, editors. Model Organisms in Drug Discovery. John Wiley & Sons; Chichester, UK: 2003. pp. 251–279.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

