PKA has a critical role in synaptic delivery of GluR1- and GluR4-containing AMPARs during initial stages of acquisition of in vitro classical conditioning
- PMID: 19261706
- PMCID: PMC2681441
- DOI: 10.1152/jn.91282.2008
PKA has a critical role in synaptic delivery of GluR1- and GluR4-containing AMPARs during initial stages of acquisition of in vitro classical conditioning
Abstract
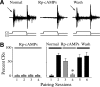
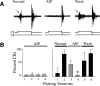
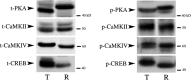
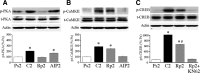
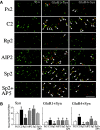
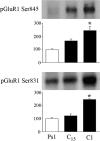
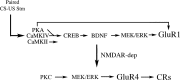
The cyclic AMP-dependent protein kinase (PKA) signaling pathway has been shown to be important in mechanisms of synaptic plasticity, although its direct and downstream signaling effects are not well understood. Using an in vitro model of eyeblink classical conditioning, we report that PKA has a critical role in initiating a signaling cascade that results in synaptic delivery of glutamate receptor 1 (GluR1)- and GluR4-containing alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) in abducens motor neurons during conditioning. PKA and the Ca(2+)-calmodulin-dependent protein kinases (CaMKs) II and IV are activated early in conditioning and are required for acquisition and expression of conditioned responses (CRs). cAMP-response-element-binding protein (CREB) is also activated early in conditioning but is blocked by coapplication of inhibitors to PKA and the CaMKs, suggesting that CREB is downstream of those signaling cascades. Moreover, evidence suggests that PKA activates extracellular signal-regulated kinase, which is also required for conditioning. Imaging studies after conditioning further indicate that colocalization of GluR1 AMPAR subunits with the synaptic marker synaptophysin requires PKA, but is insensitive to the N-methyl-d-aspartate receptor (NMDAR) inhibitor d,l-AP5. PKA activation also leads to synaptic localization of GluR4 subunits that, unlike GluR1, is dependent on NMDARs and is mediated by CaMKII. Together with previous studies, our findings support a two-stage model of AMPAR synaptic delivery during acquisition of classical conditioning. The first stage involves synaptic incorporation of GluR1-containing AMPARs that serves to activate silent synapses. This allows a second stage of NMDAR- and protein kinase C-dependent delivery of GluR4 AMPAR subunits that supports the acquisition of CRs.
Figures



Similar articles
-
Protein kinase C-dependent and independent signaling pathways regulate synaptic GluR1 and GluR4 AMPAR subunits during in vitro classical conditioning.Neuroscience. 2008 Oct 28;156(4):872-84. doi: 10.1016/j.neuroscience.2008.08.042. Epub 2008 Aug 27. Neuroscience. 2008. PMID: 18809472 Free PMC article.
-
Conversion of silent synapses into the active pool by selective GluR1-3 and GluR4 AMPAR trafficking during in vitro classical conditioning.J Neurophysiol. 2007 Sep;98(3):1278-86. doi: 10.1152/jn.00212.2007. Epub 2007 Jun 27. J Neurophysiol. 2007. PMID: 17596423
-
BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK.Neurobiol Learn Mem. 2009 Mar;91(3):243-9. doi: 10.1016/j.nlm.2008.10.002. Epub 2008 Nov 17. Neurobiol Learn Mem. 2009. PMID: 18977306 Free PMC article.
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
-
Synaptic Mechanisms of Delay Eyeblink Classical Conditioning: AMPAR Trafficking and Gene Regulation in an In Vitro Model.Mol Neurobiol. 2023 Dec;60(12):7088-7103. doi: 10.1007/s12035-023-03528-z. Epub 2023 Aug 2. Mol Neurobiol. 2023. PMID: 37531025 Review.
Cited by
-
Cloning and characterization of glutamate receptor subunit 4 (GLUA4) and its alternatively spliced isoforms in turtle brain.J Mol Neurosci. 2011 Jul;44(3):159-72. doi: 10.1007/s12031-010-9405-2. Epub 2010 Jun 15. J Mol Neurosci. 2011. PMID: 20549383 Free PMC article.
-
Oligomeric amyloid-{beta} inhibits the proteolytic conversion of brain-derived neurotrophic factor (BDNF), AMPA receptor trafficking, and classical conditioning.J Biol Chem. 2010 Nov 5;285(45):34708-17. doi: 10.1074/jbc.M110.150821. Epub 2010 Aug 31. J Biol Chem. 2010. PMID: 20807770 Free PMC article.
-
Modeling signal transduction in classical conditioning with network motifs.Front Mol Neurosci. 2011 Jul 7;4:9. doi: 10.3389/fnmol.2011.00009. eCollection 2011. Front Mol Neurosci. 2011. PMID: 21779235 Free PMC article.
-
Cleavage of proBDNF to BDNF by a tolloid-like metalloproteinase is required for acquisition of in vitro eyeblink classical conditioning.J Neurosci. 2009 Nov 25;29(47):14956-64. doi: 10.1523/JNEUROSCI.3649-09.2009. J Neurosci. 2009. PMID: 19940191 Free PMC article.
-
Co-induction of LTP and LTD and its regulation by protein kinases and phosphatases.J Neurophysiol. 2010 May;103(5):2737-46. doi: 10.1152/jn.01112.2009. Epub 2010 Mar 24. J Neurophysiol. 2010. PMID: 20457859 Free PMC article.
References
-
- Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER, Bourtchuladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615–626, 1997. - PubMed
-
- Anderson CW, Keifer J. Properties of conditioned abducens nerve responses in a highly reduced in vitro brain stem preparation from the turtle. J Neurophysiol 81: 1242–1250, 1999. - PubMed
-
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-proprionate-type glutamate receptor. J Biol Chem 272: 32727–32730, 1997. - PubMed
-
- Bolshakov VY, Golan H, Kandel ER, Siegelbaum SA. Recruitment of new sites of synaptic transmission during the cAMP-dependent late phase of LTP at CA3–CA1 synapses in the hippocampus. Neuron 19: 635–651, 1997. - PubMed
-
- Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, Carvalho AL, Esteban JA, Duarte CB. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem 282: 12619–12628, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

