Angiotensin II type 1 receptor blocker attenuates the activation of ERK and NADPH oxidase by mechanical strain in mesangial cells in the absence of angiotensin II
- PMID: 19261744
- PMCID: PMC4067119
- DOI: 10.1152/ajprenal.00580.2007
Angiotensin II type 1 receptor blocker attenuates the activation of ERK and NADPH oxidase by mechanical strain in mesangial cells in the absence of angiotensin II
Abstract
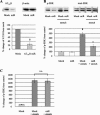
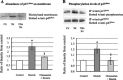
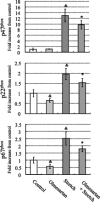
It has been reported that mechanical strain activates extracellular signal-regulated protein kinases (ERK) without the involvement of angiotensin II (Ang II) in cardiomyocytes. We examined the effects of mechanical strain on ERK phosphorylation levels in the absence of Ang II using rat mesangial cells. The ratio of phosphorylated ERK (p-ERK) to total ERK expression was increased by cyclic mechanical strain in a time- and elongation strength-dependent manner. With olmesartan [Ang II type 1 receptor (AT1R) antagonist] pretreatment, p-ERK plateau levels decreased in a dose-dependent manner (EC(50) = 1.3 x 10(-8) M, maximal inhibition 50.6 +/- 11.0% at 10(-5) M); a similar effect was observed with RNA interference against Ang II type 1A receptor (AT(1A)R) and Tempol, a superoxide dismutase mimetic. In addition to the inhibition of p-ERK levels, olmesartan blocked the increase in cell surface and phosphorylated p47(phox) induced by mechanical strain and also lowered the mRNA expression levels of NADPH oxidase subunits. These results demonstrate that mechanical strain stimulates AT1R to phosphorylate ERK in mesangial cells in the absence of Ang II. This mechanotransduction mechanism is involved in the oxidative stress caused by NADPH oxidase and is blocked by olmesartan. The inverse agonistic activity of this AT1R blocker may be useful for the prevention of mesangial proliferation and renal damage caused by mechanical strain/oxidative stress regardless of circulating or tissue Ang II levels.
Figures




Similar articles
-
Loss of biphasic effect on Na/K-ATPase activity by angiotensin II involves defective angiotensin type 1 receptor-nitric oxide signaling.Hypertension. 2008 Dec;52(6):1099-105. doi: 10.1161/HYPERTENSIONAHA.108.117911. Epub 2008 Oct 27. Hypertension. 2008. PMID: 18955661
-
Olmesartan ameliorates progressive glomerular injury in subtotal nephrectomized rats through suppression of superoxide production.Hypertens Res. 2008 Feb;31(2):305-13. doi: 10.1291/hypres.31.305. Hypertens Res. 2008. PMID: 18360051
-
Angiotensin II receptor blocker attenuates PDGF-induced mesangial cell migration in a receptor-independent manner.Nephrol Dial Transplant. 2010 Feb;25(2):364-72. doi: 10.1093/ndt/gfp520. Epub 2009 Oct 7. Nephrol Dial Transplant. 2010. PMID: 19812233
-
Role of intrarenal (pro)renin receptor in ischemic acute kidney injury in rats.Clin Exp Nephrol. 2015 Apr;19(2):185-96. doi: 10.1007/s10157-014-0979-9. Epub 2014 May 10. Clin Exp Nephrol. 2015. PMID: 24817138
-
Role of Angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension.Life Sci. 2015 Mar 1;124:81-90. doi: 10.1016/j.lfs.2015.01.005. Epub 2015 Jan 24. Life Sci. 2015. PMID: 25623850 Free PMC article.
Cited by
-
Potential dopamine-1 receptor stimulation in hypertension management.Curr Hypertens Rep. 2011 Aug;13(4):294-302. doi: 10.1007/s11906-011-0211-1. Curr Hypertens Rep. 2011. PMID: 21633929 Review.
-
Beneficial Effects of Dietary Nitrite on a Model of Nonalcoholic Steatohepatitis Induced by High-Fat/High-Cholesterol Diets in SHRSP5/Dmcr Rats: A Preliminary Study.Int J Mol Sci. 2022 Mar 8;23(6):2931. doi: 10.3390/ijms23062931. Int J Mol Sci. 2022. PMID: 35328352 Free PMC article.
-
N-type calcium channel and renal injury.Int Urol Nephrol. 2022 Nov;54(11):2871-2879. doi: 10.1007/s11255-022-03183-8. Epub 2022 Apr 13. Int Urol Nephrol. 2022. PMID: 35416563 Free PMC article. Review.
-
Aldosterone and Mineralocorticoid Receptor System in Cardiovascular Physiology and Pathophysiology.Oxid Med Cell Longev. 2018 Sep 19;2018:1204598. doi: 10.1155/2018/1204598. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30327709 Free PMC article. Review.
-
The role of endothelial mechanosensitive genes in atherosclerosis and omics approaches.Arch Biochem Biophys. 2016 Feb 1;591:111-31. doi: 10.1016/j.abb.2015.11.005. Epub 2015 Dec 11. Arch Biochem Biophys. 2016. PMID: 26686737 Free PMC article. Review.
References
-
- Ardaillou R, Chansel D, Chatziantoniou C, Dussaule JC. Mesangial AT1 receptors: expression, signaling, and regulation. J Am Soc Nephrol 10, Suppl 11: S40–S46, 1999 - PubMed
-
- Bader M, Peters J, Baltatu O, Muller DN, Luft FC, Ganten D. Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J Mol Med 79: 76–102, 2001 - PubMed
-
- Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65: 16–27, 2005 - PubMed
-
- Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471–478, 2003 - PubMed
-
- Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

