Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes
- PMID: 19261745
- PMCID: PMC2681356
- DOI: 10.1152/ajprenal.00061.2009
Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes
Abstract
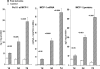
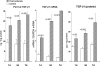
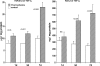
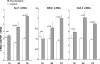
Ischemic renal injury can produce chronic renal inflammation and fibrosis. This study tested whether ischemia-reperfusion (I/R) activates histone-modifying enzyme systems and alters histone expression at selected proinflammatory/profibrotic genes. CD-1 mice were subjected to 30 min of unilateral I/R. Contralateral kidneys served as controls. At 1, 3, or 7 days of reflow, bilateral nephrectomy was performed. Renal cortices were probed for monocyte chemoattractant protein-1 (MCP-1), transforming growth factor-beta1 (TGF-beta1), and collagen III mRNAs and cytokine levels. RNA polymerase II (Pol II) binding, which initiates transcription, was quantified at exon 1 of the MCP-1, TGF-beta1, collagen III genes (chromatin immunoprecipitation assay). Two representative gene-activating histone modifications [histone 3 lysine 4 (H3K4) trimethylation (m3) (H3K4m3); histone 2 variant H2A.Z] were sought. Degrees of binding of two relevant histone-modifying enzymes (Set1, BRG1) to target genes were assessed. Renal cortical Set1, BRG1, and H2A.Z mRNAs were measured. Finally, the potential utility of urinary mRNA concentrations as noninvasive markers of these in vivo processes was tested. I/R caused progressive increases in Pol II binding to MCP-1, TGF-beta1, and collagen III genes. Parallel increases in cognate mRNAs also were expressed. Progressive increases in renal cortical Set1, BRG1, H2A.Z mRNAs, and increased Set1/BRG1 binding to target genes occurred. These changes corresponded with: 1) progressive elevations of H3K4m3 and H2A.Z at each test gene; 2) increases in renal cortical TGF-beta1/MCP-1 cytokines; and 3) renal collagen deposition (assessed by histomorphology). Postischemic increases in urinary TGF-beta1, MCP-1, Set1, and BRG1 mRNAs were also observed. We conclude that: 1) I/R upregulates histone-modifying enzyme systems, 2) histone modifications at proinflammatory/profibrotic genes can result, and 3) urinary mRNA assessments may have utility for noninvasive monitoring of these in vivo events.
Figures






Similar articles
-
Progressive histone alterations and proinflammatory gene activation: consequences of heme protein/iron-mediated proximal tubule injury.Am J Physiol Renal Physiol. 2010 Mar;298(3):F827-37. doi: 10.1152/ajprenal.00683.2009. Epub 2009 Dec 23. Am J Physiol Renal Physiol. 2010. PMID: 20032114 Free PMC article.
-
Acute unilateral ischemic renal injury induces progressive renal inflammation, lipid accumulation, histone modification, and "end-stage" kidney disease.Am J Physiol Renal Physiol. 2011 Dec;301(6):F1334-45. doi: 10.1152/ajprenal.00431.2011. Epub 2011 Sep 14. Am J Physiol Renal Physiol. 2011. PMID: 21921025 Free PMC article.
-
BRG1 increases transcription of proinflammatory genes in renal ischemia.J Am Soc Nephrol. 2009 Aug;20(8):1787-96. doi: 10.1681/ASN.2009010118. Epub 2009 Jun 25. J Am Soc Nephrol. 2009. PMID: 19556365 Free PMC article.
-
Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury.Am J Physiol Renal Physiol. 2009 Oct;297(4):F961-70. doi: 10.1152/ajprenal.00381.2009. Epub 2009 Aug 5. Am J Physiol Renal Physiol. 2009. PMID: 19656911 Free PMC article.
-
Stem Cells in Kidney Ischemia: From Inflammation and Fibrosis to Renal Tissue Regeneration.Int J Mol Sci. 2023 Feb 27;24(5):4631. doi: 10.3390/ijms24054631. Int J Mol Sci. 2023. PMID: 36902062 Free PMC article. Review.
Cited by
-
Epigenetic responses to heat: From adaptation to maladaptation.Exp Physiol. 2022 Oct;107(10):1144-1158. doi: 10.1113/EP090143. Epub 2022 May 5. Exp Physiol. 2022. PMID: 35413138 Free PMC article. Review.
-
Chromatin-modifying agents for epigenetic reprogramming and endogenous neural stem cell-mediated repair in stroke.Transl Stroke Res. 2011 Mar 1;2(1):7-16. doi: 10.1007/s12975-010-0051-3. Transl Stroke Res. 2011. PMID: 24014083 Free PMC article.
-
Epigenetics in kidney diseases.Adv Clin Chem. 2021;104:233-297. doi: 10.1016/bs.acc.2020.09.005. Epub 2020 Oct 21. Adv Clin Chem. 2021. PMID: 34462056 Free PMC article. Review.
-
Acute kidney disease: an overview of the epidemiology, pathophysiology, and management.Kidney Res Clin Pract. 2023 Nov;42(6):686-699. doi: 10.23876/j.krcp.23.001. Epub 2023 May 11. Kidney Res Clin Pract. 2023. PMID: 37165615 Free PMC article.
-
Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD.Nat Rev Nephrol. 2015 May;11(5):264-76. doi: 10.1038/nrneph.2015.3. Epub 2015 Feb 3. Nat Rev Nephrol. 2015. PMID: 25643664 Free PMC article. Review.
References
-
- Basile DP, Fredrich K, Alausa M, Vio CP, Liang M, Rieder MR, Greene AS, Cowley AW Jr. Identification of persistently altered gene expression in the kidney after functional recovery from ischemic acute renal failure. Am J Physiol Renal Physiol 288: F953–F963, 2005. - PubMed
-
- Basile DP, Martin Dr Hammerman MH. Extracellular matrix related genes in kidney postischemic injury: potential role for TGF-β1 in repair. Am J Physiol Renal Physiol 275: F894–F903, 1998. - PubMed
-
- Basile DP, Martin DR, Roethe K, Osborn J. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F884–F899, 2001. - PubMed
-
- Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas EJ III, Gingeras TR, Schreiber SL, Lander ES. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell 120: 169–181, 2005. - PubMed
-
- Border WA, Noble NA. Transforming growth factor-β in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

