Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide
- PMID: 19261856
- PMCID: PMC2660757
- DOI: 10.1073/pnas.0710416106
Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide
Abstract
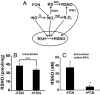
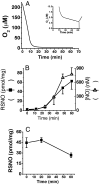
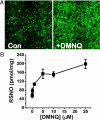
Nitrosothiols (RSNO), formed from thiols and metabolites of nitric oxide (*NO), have been implicated in a diverse set of physiological and pathophysiological processes, although the exact mechanisms by which they are formed biologically are unknown. Several candidate nitrosative pathways involve the reaction of *NO with O(2), reactive oxygen species (ROS), and transition metals. We developed a strategy using extracellular ferrocyanide to determine that under our conditions intracellular protein RSNO formation occurs from reaction of *NO inside the cell, as opposed to cellular entry of nitrosative reactants from the extracellular compartment. Using this method we found that in RAW 264.7 cells RSNO formation occurs only at very low (<8 microM) O(2) concentrations and exhibits zero-order dependence on *NO concentration. Indeed, RSNO formation is not inhibited even at O(2) levels <1 microM. Additionally, chelation of intracellular chelatable iron pool (CIP) reduces RSNO formation by >50%. One possible metal-dependent, O(2)-independent nitrosative pathway is the reaction of thiols with dinitrosyliron complexes (DNIC), which are formed in cells from the reaction of *NO with the CIP. Under our conditions, DNIC formation, like RSNO formation, is inhibited by approximately 50% after chelation of labile iron. Both DNIC and RSNO are also increased during overproduction of ROS by the redox cycler 5,8-dimethoxy-1,4-naphthoquinone. Taken together, these data strongly suggest that cellular RSNO are formed from free *NO via transnitrosation from DNIC derived from the CIP. We have examined in detail the kinetics and mechanism of RSNO formation inside cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
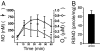

References
-
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. - PubMed
-
- Zhang Y, Hogg N. S-nitrosothiols: Cellular formation and transport. Free Radical Biol Med. 2005;38:831–838. - PubMed
-
- Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J Biol Chem. 1995;270:28158–28164. - PubMed
-
- Moller MN, Li Q, Lancaster JR, Jr, Denicola A. Acceleration of nitric oxide autoxidation and nitrosation by membranes. IUBMB Life. 2007;59:243–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

