DSCAM deficiency causes loss of pre-inspiratory neuron synchroneity and perinatal death
- PMID: 19261893
- PMCID: PMC6666194
- DOI: 10.1523/JNEUROSCI.3624-08.2009
DSCAM deficiency causes loss of pre-inspiratory neuron synchroneity and perinatal death
Abstract
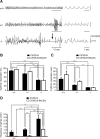
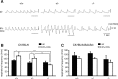
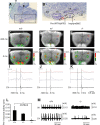
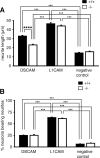
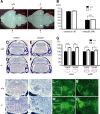
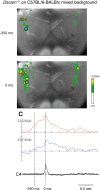
Down syndrome cell adhesion molecule (DSCAM) is a neural adhesion molecule that plays diverse roles in neural development. We disrupted the Dscam locus in mice and found that the null mutants (Dscam(-/-)) died within 24 h after birth. Whole-body plethysmography showed irregular respiration and lower ventilatory response to hypercapnia in the null mutants. Furthermore, a medulla-spinal cord preparation of Dscam(-/-) mice showed that the C4 ventral root activity, which drives diaphragm contraction for inspiration, had an irregular rhythm with frequent apneas. Optical imaging of the preparation using voltage-sensitive dye revealed that the pre-inspiratory neurons located in the rostral ventrolateral medulla and belonging to the rhythm generator for respiration, lost their synchroneity in Dscam(-/-) mice. Dscam(+/-) mice, which survived to adulthood without any overt abnormalities, also showed irregular respiration but milder than Dscam(-/-) mice. These results suggest that DSCAM plays a critical role in central respiratory regulation in a dosage-dependent manner.
Figures



References
-
- Agarwala KL, Nakamura S, Tsutsumi Y, Yamakawa K. Down syndrome cell adhesion molecule DSCAM mediates homophilic intercellular adhesion. Brain Res Mol Brain Res. 2000;79:118–126. - PubMed
-
- Agarwala KL, Ganesh S, Amano K, Suzuki T, Yamakawa K. DSCAM, a highly conserved gene in mammals, expressed in differentiating mouse brain. Biochem Biophys Res Commun. 2001;281:697–705. - PubMed
-
- Amiel J, Laudier B, Attié-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S. Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet. 2003;33:459–461. - PubMed
-
- Arata A, Onimaru H, Homma I. Respiration-related neurons in the ventral medulla of newborn rats in vitro. Brain Res Bull. 1990;24:599–604. - PubMed
-
- Barlow GM, Micales B, Chen XN, Lyons GE, Korenberg JR. Mammalian DSCAMs: roles in the development of the spinal cord, cortex, and cerebellum? Biochem Biophys Res Commun. 2002;293:881–891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
