Cell migration from baby to mother
- PMID: 19262088
- PMCID: PMC2633676
Cell migration from baby to mother
Abstract
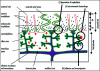
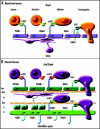
Fetal cells migrate into the mother during pregnancy. Fetomaternal transfer probably occurs in all pregnancies and in humans the fetal cells can persist for decades. Microchimeric fetal cells are found in various maternal tissues and organs including blood, bone marrow, skin and liver. In mice, fetal cells have also been found in the brain. The fetal cells also appear to target sites of injury. Fetomaternal microchimerism may have important implications for the immune status of women, influencing autoimmunity and tolerance to transplants. Further understanding of the ability of fetal cells to cross both the placental and blood-brain barriers, to migrate into diverse tissues, and to differentiate into multiple cell types may also advance strategies for intravenous transplantation of stem cells for cytotherapeutic repair. Here we discuss hypotheses for how fetal cells cross the placental and blood-brain barriers and the persistence and distribution of fetal cells in the mother.
Figures

References
-
- Adams KM, Nelson JL. Microchimerism: An investigative frontier in autoimmunity and transplantation. JAMA. 2004;291:1127–1131. - PubMed
-
- Reed W, Lee TH, Norris PJ, Utter GH, Busch MP. Transfusion-associated microchimerism: A new complication of blood transfusions in severely injured patients. Semin Hematol. 2007;44:24–31. - PubMed
-
- Iverson GM, Bianchi DW, Cann HM, Herzenberg LA. Detection and isolation of fetal cells from maternal blood using the flourescence-activated cell sorter (FACS) Prenat Diagn. 1981;1:61–73. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
