"Armed" oncolytic herpes simplex viruses for brain tumor therapy
- PMID: 19262110
- PMCID: PMC2634086
- DOI: 10.4161/cam.2.3.6353
"Armed" oncolytic herpes simplex viruses for brain tumor therapy
Abstract
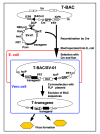
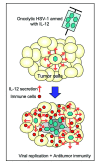
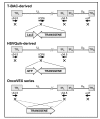
Genetically engineered, conditionally replicating herpes simplex viruses type 1 (HSV-1) are promising therapeutic agents for brain tumors and other solid cancers. They can replicate in situ, spread and exhibit oncolytic activity via a direct cytocidal effect. One of the advantages of HSV-1 is the capacity to incorporate large and/or multiple transgenes within the viral genome. Oncolytic HSV-1 can therefore be "armed" to add certain functions. Recently, the field of armed oncolytic HSV-1 has drastically advanced, due to development of recombinant HSV-1 generation systems that utilize bacterial artificial chromosome and multiple DNA recombinases. Because antitumor immunity is induced in the course of oncolytic activities of HSV-1, transgenes encoding immunomodulatory molecules have been most frequently used for arming. Other armed oncolytic HSV-1 include those that express antiangiogenic factors, fusogenic membrane glycoproteins, suicide gene products, and proapoptotic proteins. Provided that the transgene product does not interfere with viral replication, such arming of oncolytic HSV-1 results in augmentation of antitumor efficacy. Immediate-early viral promoters are often used to control the arming transgenes, but strict-late viral promoters have been shown useful to restrict the expression in the late stage of viral replication when desirable. Some armed oncolytic HSV-1 have been created for the purpose of noninvasive in vivo imaging of viral infection and replication. Development of a wide variety of armed oncolytic HSV-1 will lead to an establishment of a new genre of therapy for brain tumors as well as other cancers.
Figures
Similar articles
-
Active immunotherapy: oncolytic virus therapy using HSV-1.Adv Exp Med Biol. 2012;746:178-86. doi: 10.1007/978-1-4614-3146-6_14. Adv Exp Med Biol. 2012. PMID: 22639168 Review.
-
Oncolytic virus therapy using genetically engineered herpes simplex viruses.Front Biosci. 2008 Jan 1;13:2060-4. doi: 10.2741/2823. Front Biosci. 2008. PMID: 17981691 Review.
-
Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system.Cancer Res. 2005 Dec 1;65(23):10663-8. doi: 10.1158/0008-5472.CAN-05-2534. Cancer Res. 2005. PMID: 16322208
-
The potential application of a transcriptionally regulated oncolytic herpes simplex virus for human cancer therapy.Br J Cancer. 2014 Jan 7;110(1):94-106. doi: 10.1038/bjc.2013.692. Epub 2013 Nov 5. Br J Cancer. 2014. PMID: 24196790 Free PMC article.
-
Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7-1 results in enhanced antitumor efficacy.Clin Cancer Res. 2006 Jan 15;12(2):643-52. doi: 10.1158/1078-0432.CCR-05-1494. Clin Cancer Res. 2006. PMID: 16428511
Cited by
-
Oncolytic Virus Encoding a Master Pro-Inflammatory Cytokine Interleukin 12 in Cancer Immunotherapy.Cells. 2020 Feb 10;9(2):400. doi: 10.3390/cells9020400. Cells. 2020. PMID: 32050597 Free PMC article. Review.
-
Oncolytic Herpes Simplex Viral Therapy: A Stride toward Selective Targeting of Cancer Cells.Front Pharmacol. 2017 May 16;8:270. doi: 10.3389/fphar.2017.00270. eCollection 2017. Front Pharmacol. 2017. PMID: 28559846 Free PMC article. Review.
-
Molecular imaging with bioluminescence and PET reveals viral oncolysis kinetics and tumor viability.Cancer Res. 2014 Aug 1;74(15):4111-21. doi: 10.1158/0008-5472.CAN-13-3472. Epub 2014 May 29. Cancer Res. 2014. PMID: 24876106 Free PMC article.
-
Gene Therapy Tools for Brain Diseases.Front Pharmacol. 2019 Jul 1;10:724. doi: 10.3389/fphar.2019.00724. eCollection 2019. Front Pharmacol. 2019. PMID: 31312139 Free PMC article. Review.
-
Using viral vectors as gene transfer tools (Cell Biology and Toxicology Special Issue: ETCS-UK 1 day meeting on genetic manipulation of cells).Cell Biol Toxicol. 2010 Feb;26(1):1-20. doi: 10.1007/s10565-009-9139-5. Epub 2009 Oct 15. Cell Biol Toxicol. 2010. PMID: 19830583 Free PMC article.
References
-
- Aghi M, Martuza RL. Oncolytic viral therapies—the clinical experience. Oncogene. 2005;24:7802–7816. - PubMed
-
- Todo T. Oncolytic virus therapy using genetically engineered herpes simplex viruses. Front Biosci. 2008;13:2060–2064. - PubMed
-
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, Palmer CA, Feigenbaum F, Tornatore C, Tufaro F, Martuza RL. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. [see comments] - PubMed
-
- Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. - PubMed
-
- Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 34.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
