MEP1A allele for meprin A metalloprotease is a susceptibility gene for inflammatory bowel disease
- PMID: 19262505
- PMCID: PMC2670347
- DOI: 10.1038/mi.2009.3
MEP1A allele for meprin A metalloprotease is a susceptibility gene for inflammatory bowel disease
Abstract
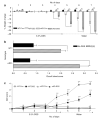
The MEP1A gene, located on human chromosome 6p (mouse chromosome 17) in a susceptibility region for inflammatory bowel disease (IBD), encodes the alpha-subunit of metalloproteinase meprin A, which is expressed in the intestinal epithelium. This study shows a genetic association of MEP1A with IBD in a cohort of ulcerative colitis (UC) patients. There were four single-nucleotide polymorphisms in the coding region (P=0.0012-0.04), and one in the 3'-untranslated region (P=2 x 10(-7)) that displayed associations with UC. Moreover, meprin-alpha mRNA was decreased in inflamed mucosa of IBD patients. Meprin-alpha knockout mice exhibited a more severe intestinal injury and inflammation than their wild-type counterparts following oral administration of dextran sulfate sodium. Collectively, the data implicate MEP1A as a UC susceptibility gene and indicate that decreased meprin-alpha expression is associated with intestinal inflammation in IBD patients and in a mouse experimental model of IBD.
Figures
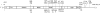




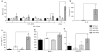
References
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
-
- Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. - PubMed
-
- Jiang W, et al. Fine mapping of MEP1A, the gene encoding the alpha subunit of the metalloendopeptidase meprin, to human chromosome 6P21. Biochem. Biophys. Res. Commun. 1995;216:630–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

