A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence
- PMID: 19262506
- PMCID: PMC3036970
- DOI: 10.1038/mi.2009.4
A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence
Abstract
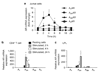
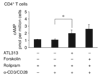
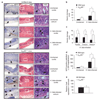
Helicobacter pylori causes a lifelong infection and provides a model of bacterial adaptation and persistent colonization. Adenosine is an anti-inflammatory mediator that limits tissue damage during inflammation. We studied the role of adenosine in the T-cell-mediated regulation of gastritis and bacterial persistence. After 4 h of activation, human T helper (Th) cells increased A(2A) adenosine receptor (A(2A)AR) mRNA level (sevenfold). A(2A)AR was the predominant subtype expressed in resting and stimulated gastric or peripheral Th cells. Stimulation with ATL313, an A(2A)AR agonist, increased cyclic AMP (cAMP) accumulation and reduced interleukin-2 (IL-2) production by 20-50%. ATL313 also attenuated tumor necrosis factor-alpha (TNF-alpha) and interferon-gamma (IFN-gamma) production, which was inhibited by an A(2A)AR antagonist. Infection of IL-10-deficient mice with H. pylori is cleared spontaneously due to the marked inflammation. Administration of ATL313 during infection reduced gastritis and pro-inflammatory cytokine responses while bacterial load increased. In contrast, infection of A(2A)AR-deficient mice enhanced gastritis. Thus, A(2A)AR limits the pro-inflammatory effects of Th cells and favor chronic Helicobacter infection.
Figures





References
-
- Dixon MF. Pathophysiology of Helicobacter pylori infection. Scand. J. Gastroenterol. 1994;29:7–10. - PubMed
-
- Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244–1252. - PubMed
-
- Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. - PubMed
-
- Correa P, Miller MJS. Carcinogenesis, apoptosis and cell proliferation. Br. Med. Bull. 1998;54:151–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

