Profilin 1 is required for abscission during late cytokinesis of chondrocytes
- PMID: 19262563
- PMCID: PMC2683706
- DOI: 10.1038/emboj.2009.58
Profilin 1 is required for abscission during late cytokinesis of chondrocytes
Abstract
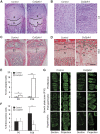
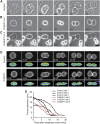
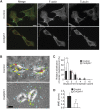
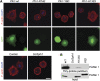
Profilins are key factors for dynamic rearrangements of the actin cytoskeleton. However, the functions of profilins in differentiated mammalian cells are uncertain because profilin deficiency is early embryonic lethal for higher eukaryotes. To examine profilin function in chondrocytes, we disrupted the profilin 1 gene in cartilage (Col2pfn1). Homozygous Col2pfn1 mice develop progressive chondrodysplasia caused by disorganization of the growth plate and defective chondrocyte cytokinesis, indicated by the appearance of binucleated cells. Surprisingly, Col2pfn1 chondrocytes assemble and contract actomyosin rings normally during cell division; however, they display defects during late cytokinesis as they frequently fail to complete abscission due to their inability to develop strong traction forces. This reduced force generation results from an impaired formation of lamellipodia, focal adhesions and stress fibres, which in part could be linked to an impaired mDia1-mediated actin filament elongation. Neither an actin nor a poly-proline binding-deficient profilin 1 is able to rescue the defects. Taken together, our results demonstrate that profilin 1 is not required for actomyosin ring formation in dividing chondrocytes but necessary to generate sufficient force for abscission during late cytokinesis.
Figures



References
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246–20249 - PubMed
-
- Betz UA, Vosshenrich CA, Rajewsky K, Müller W (1996) Bypass of lethality with mosaic mice generated by Cre-loxP-mediated recombination. Curr Biol 6: 1307–1316 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

