Phosphodiesterase 3B is localized in caveolae and smooth ER in mouse hepatocytes and is important in the regulation of glucose and lipid metabolism
- PMID: 19262749
- PMCID: PMC2650791
- DOI: 10.1371/journal.pone.0004671
Phosphodiesterase 3B is localized in caveolae and smooth ER in mouse hepatocytes and is important in the regulation of glucose and lipid metabolism
Abstract
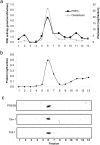
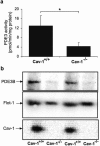
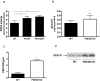
Cyclic nucleotide phosphodiesterases (PDEs) are important regulators of signal transduction processes mediated by cAMP and cGMP. One PDE family member, PDE3B, plays an important role in the regulation of a variety of metabolic processes such as lipolysis and insulin secretion. In this study, the cellular localization and the role of PDE3B in the regulation of triglyceride, cholesterol and glucose metabolism in hepatocytes were investigated. PDE3B was identified in caveolae, specific regions in the plasma membrane, and smooth endoplasmic reticulum. In caveolin-1 knock out mice, which lack caveolae, the amount of PDE3B protein and activity were reduced indicating a role of caveolin-1/caveolae in the stabilization of enzyme protein. Hepatocytes from PDE3B knock out mice displayed increased glucose, triglyceride and cholesterol levels, which was associated with increased expression of gluconeogenic and lipogenic genes/enzymes including, phosphoenolpyruvate carboxykinase, peroxisome proliferator-activated receptor gamma, sterol regulatory element-binding protein 1c and hydroxyl-3-methylglutaryl coenzyme A reductase. In conclusion, hepatocyte PDE3B is localized in caveolae and smooth endoplasmic reticulum and plays important roles in the regulation of glucose, triglyceride and cholesterol metabolism. Dysregulation of PDE3B could have a role in the development of fatty liver, a condition highly relevant in the context of type 2 diabetes.
Conflict of interest statement
Figures

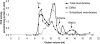

References
-
- Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. - PubMed
-
- Degerman E, Manganiello V. Phosphodiesterase 3B; an important regulator of energy homeostasis. In: Beavo, Houslay, Frances, editors. Cyclic Phosphodiesterases in health and disease. Broca Raton, FL USA: CRC Press; 2007. pp. 79–99.
-
- Thompson PE, Manganiello V, Degerman E. Re-discovering PDE3 inhibitors–new opportunities for a long neglected target. Curr Top Med Chem. 2007;7(4):421–36. - PubMed
-
- Nilsson R, Ahmad F, Swärd K, Andersson U, Weston M, et al. Plasma membrane cyclic nucleotide phosphodiesterase 3B (PDE3B) is associated with caveolae in primary adipocytes. Cell Signal. 2006;18(10):1713–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

