Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya
- PMID: 19262754
- PMCID: PMC2650803
- DOI: 10.1371/journal.pone.0004708
Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya
Abstract
Objective: The antigen, falciparum malaria protein 1 (FMP1), represents the 42-kDa C-terminal fragment of merozoite surface protein-1 (MSP-1) of the 3D7 clone of P. falciparum. Formulated with AS02 (a proprietary Adjuvant System), it constitutes the FMP1/AS02 candidate malaria vaccine. We evaluated this vaccine's safety, immunogenicity, and efficacy in African children.
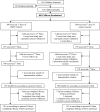
Methods: A randomised, double-blind, Phase IIb, comparator-controlled trial.The trial was conducted in 13 field stations of one mile radii within Kombewa Division, Nyanza Province, Western Kenya, an area of holoendemic transmission of P. falciparum. We enrolled 400 children aged 12-47 months in general good health.Children were randomised in a 1ratio1 fashion to receive either FMP1/AS02 (50 microg) or Rabipur(R) rabies vaccine. Vaccinations were administered on a 0, 1, and 2 month schedule. The primary study endpoint was time to first clinical episode of P. falciparum malaria (temperature >/=37.5 degrees C with asexual parasitaemia of >/=50,000 parasites/microL of blood) occurring between 14 days and six months after a third dose. Case detection was both active and passive. Safety and immunogenicity were evaluated for eight months after first immunisations; vaccine efficacy (VE) was measured over a six-month period following third vaccinations.
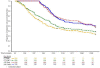
Results: 374 of 400 children received all three doses and completed six months of follow-up. FMP1/AS02 had a good safety profile and was well-tolerated but more reactogenic than the comparator. Geometric mean anti-MSP-1(42) antibody concentrations increased from1.3 microg/mL to 27.3 microg/mL in the FMP1/AS02 recipients, but were unchanged in controls. 97 children in the FMP1/AS02 group and 98 controls had a primary endpoint episode. Overall VE was 5.1% (95% CI: -26% to +28%; p-value = 0.7).
Conclusions: FMP1/AS02 is not a promising candidate for further development as a monovalent malaria vaccine. Future MSP-1(42) vaccine development should focus on other formulations and antigen constructs.
Trial registration: Clinicaltrials.gov NCT00223990.
Conflict of interest statement
Figures


References
-
- Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64(1–2 Suppl):85–96. - PubMed
-
- Ballou WR, Arevalo-Herrera M, Carucci D, Richie TL, Corradin G, et al. Update on the clinical development of candidate malaria vaccines. Am J Trop Med Hyg. 2004;71(2 Suppl):239–247. - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Amanda L, Macete E, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised, controlled trial. The Lancet. 2004;364:1411–1420. - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Amanda L, Macete E, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. The Lancet. 2005;366:2012–2018. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

