PKR acts early in infection to suppress Semliki Forest virus production and strongly enhances the type I interferon response
- PMID: 19264662
- PMCID: PMC2885058
- DOI: 10.1099/vir.0.007336-0
PKR acts early in infection to suppress Semliki Forest virus production and strongly enhances the type I interferon response
Abstract
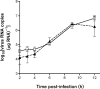
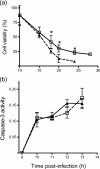
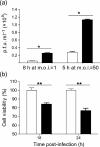
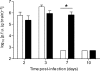
The double-stranded RNA-activated protein kinase (PKR) is a key regulator of protein translation, interferon (IFN) expression and cell survival. Upon infection of vertebrate cells in continuous culture, the alphavirus Semliki Forest virus (SFV) initiates apoptosis and IFN synthesis. To determine the effect of PKR on SFV infection, we studied the course of infection in wild-type (wt) mice, mice with a genetic deletion of PKR (PKR-/-) and mouse embryo fibroblasts (MEFs) derived from these mice. In MEFs, PKR delayed virus protein synthesis, production of infectious virus and caspase-3-activated cell death and reduced the yield of infectious virus by 90%. Small interfering RNA suppression of PKR levels in NIH-3T3 cells also reduced virus production and apoptosis. In MEFs, PKR was not required for initiation of IFN-beta gene transcription, but contributed strongly to the magnitude of this response. Levels of IFN-beta transcripts in PKR-/- MEFs at 8 h were 80% lower than those in wt MEFs and levels of functional IFN at 24 h were 95% lower. Following infection of wt and PKR-/- mice, SFV4 and SFV A7(74) were avirulent. PKR increased levels of serum IFN and the rate of clearance of infectious virus from the brain. In summary, in response to SFV, PKR exerts an early antiviral effect that delays virus protein production and release of infectious virus and, whilst PKR is not required for induction of apoptosis or activation of the type I IFN response, it strongly augments the type I IFN response and contributes to clearance of infectious virus from the mouse brain.
Figures




References
-
- Abraham, N., Stojdl, D. F., Duncan, P. I., Methot, N., Ishii, T., Dube, M., Vanderhyden, B. C., Atkins, H. L., Gray, D. A. & other authors (1999). Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem 274, 5953–5962. - PubMed
-
- Amor, S., Scallan, M. F., Morris, M. M., Dyson, H. & Fazakerley, J. K. (1996). Role of immune responses in protection and pathogenesis during Semliki Forest virus encephalitis. J Gen Virol 77, 281–291. - PubMed
-
- Baltzis, D., Li, S. & Koromilas, A. E. (2002). Functional characterization of pkr gene products expressed in cells from mice with a targeted deletion of the N terminus or C terminus domain of PKR. J Biol Chem 277, 38364–38372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

