Mepolizumab and exacerbations of refractory eosinophilic asthma
- PMID: 19264686
- PMCID: PMC3992367
- DOI: 10.1056/NEJMoa0808991
Mepolizumab and exacerbations of refractory eosinophilic asthma
Erratum in
- N Engl J Med. 2011 Feb 10;364(6):588
Abstract
Background: Exacerbations of asthma are associated with substantial morbidity and mortality and with considerable use of health care resources. Preventing exacerbations remains an important goal of therapy. There is evidence that eosinophilic inflammation of the airway is associated with the risk of exacerbations.
Methods: We conducted a randomized, double-blind, placebo-controlled, parallel-group study of 61 subjects who had refractory eosinophilic asthma and a history of recurrent severe exacerbations. Subjects received infusions of either mepolizumab, an anti-interleukin-5 monoclonal antibody (29 subjects), or placebo (32) at monthly intervals for 1 year. The primary outcome measure was the number of severe exacerbations per subject during the 50-week treatment phase. Secondary outcomes included a change in asthma symptoms, scores on the Asthma Quality of Life Questionnaire (AQLQ, in which scores range from 1 to 7, with lower values indicating more severe impairment and a change of 0.5 unit considered to be clinically important), forced expiratory volume in 1 second (FEV(1)) after use of a bronchodilator, airway hyperresponsiveness, and eosinophil counts in the blood and sputum.
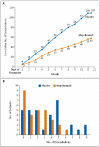
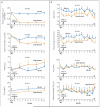
Results: Mepolizumab was associated with significantly fewer severe exacerbations than placebo over the course of 50 weeks (2.0 vs. 3.4 mean exacerbations per subject; relative risk, 0.57; 95% confidence interval [CI], 0.32 to 0.92; P=0.02) and with a significant improvement in the score on the AQLQ (mean increase from baseline, 0.55 vs. 0.19; mean difference between groups, 0.35; 95% CI, 0.08 to 0.62; P=0.02). Mepolizumab significantly lowered eosinophil counts in the blood (P<0.001) and sputum (P=0.002). There were no significant differences between the groups with respect to symptoms, FEV(1) after bronchodilator use, or airway hyperresponsiveness. The only serious adverse events reported were hospitalizations for acute severe asthma.
Conclusions: Mepolizumab therapy reduces exacerbations and improves AQLQ scores in patients with refractory eosinophilic asthma. The results of our study suggest that eosinophils have a role as important effector cells in the pathogenesis of severe exacerbations of asthma in this patient population. (Current Controlled Trials number, ISRCTN75169762.)
2009 Massachusetts Medical Society
Figures
Comment in
-
Eosinophils in asthma--closing the loop or opening the door?N Engl J Med. 2009 Mar 5;360(10):1026-8. doi: 10.1056/NEJMe0900334. N Engl J Med. 2009. PMID: 19264692 No abstract available.
-
Mepolizumab for difficult-to-control asthma with persistent sputum eosinophilia.Expert Opin Investig Drugs. 2009 Jun;18(6):869-71. doi: 10.1517/13543780902922678. Expert Opin Investig Drugs. 2009. PMID: 19426126
-
Anti-interleukin-5 therapy and severe asthma.N Engl J Med. 2009 Jun 11;360(24):2576; author reply 2577. doi: 10.1056/NEJMc090685. N Engl J Med. 2009. PMID: 19516040 No abstract available.
-
Anti-interleukin-5 therapy and severe asthma.N Engl J Med. 2009 Jun 11;360(24):2576-7; author reply 2577. N Engl J Med. 2009. PMID: 19522046 No abstract available.
-
Anti-interleukin-5 therapy and severe asthma.N Engl J Med. 2009 Jun 11;360(24):2577; author reply 2578. N Engl J Med. 2009. PMID: 19522047 No abstract available.
References
-
- Global Initiative for Asthma (GINA) [Accessed February 9, 2009];Global strategy for asthma management and prevention. 2007 http://www.ginasthma.org
-
- Jatakanon A, Lim S, Barnes PJ. Changes in sputum eosinophils predict loss of asthma control. Am J Respir Crit Care Med. 2000;161:64–72. - PubMed
-
- Deykin A, Lazarus SC, Fahy JV, et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J Allergy Clin Immunol. 2005;115:720–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
