Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways
- PMID: 19264701
- PMCID: PMC3093983
- DOI: 10.1095/biolreprod.108.074898
Prepubertal primordial follicle loss in mice is not due to classical apoptotic pathways
Abstract
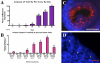
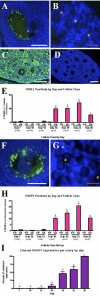
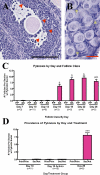
More than half of the primordial follicles that are formed by Day 6 of postnatal life in the mouse will be eliminated from the ovary by the time of puberty. Apoptosis, a form of programmed cell death, is one mechanism by which these follicles could be actively lost. To investigate whether apoptosis is responsible for the loss of primordial follicles, follicular atresia was examined during the prepubertal period, when follicles die and are cleared from the ovary at an extremely high rate. Four hallmarks of classical apoptosis were measured in follicles present in prepubertal ovaries. The primordial follicle cohort was not positively associated with nuclear condensation or cell shrinkage, activation of caspase 3, cleavage of poly(ADP ribose) polymerase 1 (PARP1), or fragmentation of DNA. These data are consistent with a nonapoptotic pathway that is responsible for small follicle death.
Figures

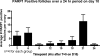
Similar articles
-
Evidence of apoptosis as an early event leading to cyclophosphamide-induced primordial follicle depletion in a prepubertal mouse model.Front Endocrinol (Lausanne). 2024 Oct 14;15:1322592. doi: 10.3389/fendo.2024.1322592. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39469582 Free PMC article.
-
Janus kinase JAK1 maintains the ovarian reserve of primordial follicles in the mouse ovary.Mol Hum Reprod. 2018 Nov 1;24(11):533-542. doi: 10.1093/molehr/gay041. Mol Hum Reprod. 2018. PMID: 30247637
-
BAX is involved in regulating follicular growth, but is dispensable for follicle atresia in adult mouse ovaries.Reproduction. 2007 Jan;133(1):107-16. doi: 10.1530/REP-06-0144. Reproduction. 2007. PMID: 17244737
-
Cellular and molecular regulation of the activation of mammalian primordial follicles: somatic cells initiate follicle activation in adulthood.Hum Reprod Update. 2015 Nov-Dec;21(6):779-86. doi: 10.1093/humupd/dmv037. Epub 2015 Jul 30. Hum Reprod Update. 2015. PMID: 26231759 Review.
-
The primordial pool of follicles and nest breakdown in mammalian ovaries.Mol Hum Reprod. 2009 Dec;15(12):795-803. doi: 10.1093/molehr/gap073. Epub 2009 Aug 26. Mol Hum Reprod. 2009. PMID: 19710243 Free PMC article. Review.
Cited by
-
Prenatal exposure to chromium induces early reproductive senescence by increasing germ cell apoptosis and advancing germ cell cyst breakdown in the F1 offspring.Dev Biol. 2014 Apr 1;388(1):22-34. doi: 10.1016/j.ydbio.2014.02.003. Epub 2014 Feb 12. Dev Biol. 2014. PMID: 24530425 Free PMC article.
-
Dacarbazine depletes the ovarian reserve in mice and depletion is enhanced with age.Sci Rep. 2018 Apr 25;8(1):6516. doi: 10.1038/s41598-018-24960-5. Sci Rep. 2018. PMID: 29695822 Free PMC article.
-
Simultaneous gene deletion of gata4 and gata6 leads to early disruption of follicular development and germ cell loss in the murine ovary.Biol Reprod. 2014 Jul;91(1):24. doi: 10.1095/biolreprod.113.117002. Epub 2014 Jun 4. Biol Reprod. 2014. PMID: 24899573 Free PMC article.
-
Oogenesis and cell death in human prenatal ovaries: what are the criteria for oocyte selection?Mol Hum Reprod. 2009 Dec;15(12):805-19. doi: 10.1093/molehr/gap055. Epub 2009 Jul 7. Mol Hum Reprod. 2009. PMID: 19584195 Free PMC article.
-
Acute Exposure to Di(2-Ethylhexyl) Phthalate in Adulthood Causes Adverse Reproductive Outcomes Later in Life and Accelerates Reproductive Aging in Female Mice.Toxicol Sci. 2016 Mar;150(1):97-108. doi: 10.1093/toxsci/kfv317. Epub 2015 Dec 16. Toxicol Sci. 2016. PMID: 26678702 Free PMC article.
References
-
- Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev 1994; 15: 707 724 - PubMed
-
- Coucouvanis EC, Sherwood SW, Carswell-Crumpton C, Spack EG, Jones PP. Evidence that the mechanism of prenatal germ cell death in the mouse is apoptosis. Exp Cell Res 1993; 209: 238 247 - PubMed
-
- Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 2001; 234: 339 351 - PubMed
-
- Pesce M, De Felici M. Apoptosis in mouse primordial germ cells: a study by transmission and scanning electron microscope. Anat Embryol (Berl) 1994; 189: 435 440 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

