NF-kappaB p50 plays distinct roles in the establishment and control of murine gammaherpesvirus 68 latency
- PMID: 19264770
- PMCID: PMC2682087
- DOI: 10.1128/JVI.00111-09
NF-kappaB p50 plays distinct roles in the establishment and control of murine gammaherpesvirus 68 latency
Abstract
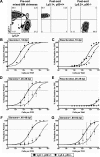
NF-kappaB signaling is critical to the survival and transformation of cells infected by the human gammaherpesviruses Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus. Here we have examined how elimination of the NF-kappaB transcription factor p50 from mice affects the life cycle of murine gammaherpesvirus 68 (MHV68). Notably, mice lacking p50 in every cell type were unable to establish a sufficiently robust immune response to control MHV68 infection, leading to high levels of latently infected B cells detected in the spleen and persistent virus replication in the lungs. The latter correlated with very low levels of virus-specific immunoglobulin G (IgG) in the infected p50(-/-) mice at day 48 postinfection. Because the confounding impact of the loss of p50 on the host response to MHV68 infection prevented a direct analysis of the role of this NF-kappaB family member on MHV68 latency in B cells, we generated and infected mixed p50(+/+)/p50(-/-) bone marrow chimeric mice. We show that the chimeric mice were able to control acute virus replication and exhibited normal levels of virus-specific IgG at 3 months postinfection, indicating the induction of a normal host immune response to MHV68 infection. However, in p50(+/+)/p50(-/-) chimeric mice the p50(-/-) B cells exhibited a significant defect compared to p50(+/+) B cells in supporting MHV68 latency. In addition to identifying a role for p50 in the establishment of latency, we determined that the absence of p50 in a subset of the hematopoietic compartment led to persistent virus replication in the lungs of the chimeric mice, providing evidence that p50 is required for controlling virus reactivation. Taken together, these data demonstrate that p50 is required for immune control by the host and has distinct tissue-dependent roles in the regulation of murine gammaherpesvirus latency during chronic infection.
Figures




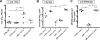


Similar articles
-
Role for MyD88 signaling in murine gammaherpesvirus 68 latency.J Virol. 2008 Apr;82(8):3853-63. doi: 10.1128/JVI.02577-07. Epub 2008 Feb 6. J Virol. 2008. PMID: 18256152 Free PMC article.
-
Establishment and maintenance of long-term murine gammaherpesvirus 68 latency in B cells in the absence of CD40.J Virol. 2005 Mar;79(5):2891-9. doi: 10.1128/JVI.79.5.2891-2899.2005. J Virol. 2005. PMID: 15709008 Free PMC article.
-
Blimp-1-dependent plasma cell differentiation is required for efficient maintenance of murine gammaherpesvirus latency and antiviral antibody responses.J Virol. 2010 Jan;84(2):674-85. doi: 10.1128/JVI.01306-09. Epub 2009 Nov 4. J Virol. 2010. PMID: 19889763 Free PMC article.
-
Interplay of Murine Gammaherpesvirus 68 with NF-kappaB Signaling of the Host.Front Microbiol. 2016 Aug 17;7:1202. doi: 10.3389/fmicb.2016.01202. eCollection 2016. Front Microbiol. 2016. PMID: 27582728 Free PMC article. Review.
-
Pathogenesis and host control of gammaherpesviruses: lessons from the mouse.Annu Rev Immunol. 2011;29:351-97. doi: 10.1146/annurev-immunol-072710-081639. Annu Rev Immunol. 2011. PMID: 21219186 Review.
Cited by
-
Tiled microarray identification of novel viral transcript structures and distinct transcriptional profiles during two modes of productive murine gammaherpesvirus 68 infection.J Virol. 2012 Apr;86(8):4340-57. doi: 10.1128/JVI.05892-11. Epub 2012 Feb 8. J Virol. 2012. PMID: 22318145 Free PMC article.
-
Murine gamma-herpesvirus 68 hijacks MAVS and IKKbeta to initiate lytic replication.PLoS Pathog. 2010 Jul 29;6(7):e1001001. doi: 10.1371/journal.ppat.1001001. PLoS Pathog. 2010. PMID: 20686657 Free PMC article.
-
Identification of alternative transcripts encoding the essential murine gammaherpesvirus lytic transactivator RTA.J Virol. 2014 May;88(10):5474-90. doi: 10.1128/JVI.03110-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574412 Free PMC article.
-
CD154:CD11b blockade enhances CD8+ T cell differentiation during infection but not transplantation.JCI Insight. 2025 Jun 9;10(11):e184843. doi: 10.1172/jci.insight.184843. eCollection 2025 Jun 9. JCI Insight. 2025. PMID: 40485581 Free PMC article.
-
Ataxia telangiectasia mutated kinase controls chronic gammaherpesvirus infection.J Virol. 2012 Dec;86(23):12826-37. doi: 10.1128/JVI.00917-12. Epub 2012 Sep 19. J Virol. 2012. PMID: 22993144 Free PMC article.
References
-
- An, J., Y. Sun, R. Sun, and M. B. Rettig. 2003. Kaposi's sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-kappaB and JNK/AP1 pathways. Oncogene 223371-3385. - PubMed
-
- Artis, D., K. Speirs, K. Joyce, M. Goldschmidt, J. Caamaño, C. A. Hunter, and P. Scott. 2003. NF-kappa B1 is required for optimal CD4+ Th1 cell development and resistance to Leishmania major. J. Immunol. 1701995-2003. - PubMed
-
- Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385347-350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

