Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans
- PMID: 19264868
- PMCID: PMC2689806
- DOI: 10.1210/en.2008-1575
Excessive ovarian production of nerve growth factor facilitates development of cystic ovarian morphology in mice and is a feature of polycystic ovarian syndrome in humans
Abstract
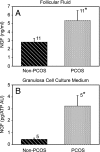
Although ovarian nerve growth factor (NGF) facilitates follicular development and ovulation, an excess of the neurotrophin in the rodent ovary reduces ovulatory capacity and causes development of precystic follicles. Here we show that ovarian NGF production is enhanced in patients with polycystic ovarian syndrome (PCOS) and that transgenically driven overproduction of NGF targeted to the ovary results in cystic morphology, when accompanied by elevated LH levels. NGF levels are increased in the follicular fluid from PCOS ovaries and in the culture medium of granulosa cells from PCOS patients, as compared with non-PCOS patients. Ovaries from transgenic mice carrying the NGF gene targeted to thecal-interstitial cells by the 17alpha-hydroxylase gene promoter produce more NGF than wild-type (WT) ovaries and are hyperinnervated by sympathetic nerves. Antral follicle growth is arrested resulting in accumulation of intermediate size follicles, many of which are apoptotic. Peripubertal transgenic mice respond to a gonadotropin challenge with a greater increase in plasma 17-hydroxyprogesterone, estradiol, and testosterone levels than WT controls. Transgenic mice also exhibit a reduced ovulatory response, delayed puberty, and reduced fertility, as assessed by a prolonged interval between litters, and a reduced number of pups per litter. Sustained, but mild, elevation of plasma LH levels results in a heightened incidence of ovarian follicular cysts in transgenic mice as compared with WT controls. These results suggest that overproduction of ovarian NGF is a component of polycystic ovarian morphology in both humans and rodents and that a persistent elevation in plasma LH levels is required for the morphological abnormalities to appear.
Figures





Similar articles
-
Excessive ovarian production of nerve growth factor elicits granulosa cell apoptosis by setting in motion a tumor necrosis factor α/stathmin-mediated death signaling pathway.Reproduction. 2011 Aug;142(2):319-31. doi: 10.1530/REP-11-0134. Epub 2011 Jun 6. Reproduction. 2011. PMID: 21646391 Free PMC article.
-
Excess of nerve growth factor in the ovary causes a polycystic ovary-like syndrome in mice, which closely resembles both reproductive and metabolic aspects of the human syndrome.Endocrinology. 2014 Nov;155(11):4494-506. doi: 10.1210/en.2014-1368. Epub 2014 Sep 11. Endocrinology. 2014. PMID: 25211588 Free PMC article.
-
Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-alpha knockout mouse.Endocrinology. 1999 Dec;140(12):5855-65. doi: 10.1210/endo.140.12.7222. Endocrinology. 1999. PMID: 10579351
-
Steroidogenesis in human polycystic ovary.Endocrinol Metab Clin North Am. 1988 Dec;17(4):751-69. Endocrinol Metab Clin North Am. 1988. PMID: 3143567 Review.
-
Circulating and ovarian IGF binding proteins: potential roles in normo-ovulatory cycles and in polycystic ovarian syndrome.Prog Growth Factor Res. 1995;6(2-4):397-408. doi: 10.1016/0955-2235(95)00016-x. Prog Growth Factor Res. 1995. PMID: 8817683 Review.
Cited by
-
CUMS Promotes the Development of Premature Ovarian Insufficiency Mediated by Nerve Growth Factor and Its Receptor in Rats.Biomed Res Int. 2020 Jun 30;2020:1946853. doi: 10.1155/2020/1946853. eCollection 2020. Biomed Res Int. 2020. PMID: 32685448 Free PMC article.
-
Methylation patterns of Brahma during spermatogenesis and oogenesis: potential implications.Fertil Steril. 2011 Jan;95(1):382-4. doi: 10.1016/j.fertnstert.2010.05.064. Epub 2010 Aug 17. Fertil Steril. 2011. PMID: 20719309 Free PMC article.
-
Circadian Rhythms Within the Female HPG Axis: From Physiology to Etiology.Endocrinology. 2021 Aug 1;162(8):bqab117. doi: 10.1210/endocr/bqab117. Endocrinology. 2021. PMID: 34125877 Free PMC article. Review.
-
PCOS Forum: research in polycystic ovary syndrome today and tomorrow.Clin Endocrinol (Oxf). 2011 Apr;74(4):424-33. doi: 10.1111/j.1365-2265.2010.03956.x. Clin Endocrinol (Oxf). 2011. PMID: 21158892 Free PMC article. Review.
-
Increased expression of circulating miRNA-93 in women with polycystic ovary syndrome may represent a novel, non-invasive biomarker for diagnosis.Sci Rep. 2015 Nov 19;5:16890. doi: 10.1038/srep16890. Sci Rep. 2015. PMID: 26582398 Free PMC article.
References
-
- Ehrmann DA 2005 Polycystic ovary syndrome. N Engl J Med 352:1223–1236 - PubMed
-
- Norman RJ, Dewailly D, Legro RS, Hickey TE 2007 Polycystic ovary syndrome. Lancet 370:685–697 - PubMed
-
- Hall JE, Taylor AE, Hayes FJ, Crowley Jr WF 1998 Insights into hypothalamic-pituitary dysfunction in polycystic ovary syndrome. J Endocrinol Invest 21:602–611 - PubMed
-
- McCartney CR, Eagleson CA, Marshall JC 2002 Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med 20:317–326 - PubMed
-
- Dunaif A, Thomas A 2001 Current concepts in the polycystic ovary syndrome. Annu Rev Med 52:401–419 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

