Maturation of the fetal human choriocapillaris
- PMID: 19264887
- PMCID: PMC4941237
- DOI: 10.1167/iovs.08-2614
Maturation of the fetal human choriocapillaris
Abstract
Purpose: The purpose of this study was to examine the structural and functional maturation of the choriocapillaris (CC) and to determine when fenestrations form, the capillaries are invested with pericytes, and the endothelial cells (ECs) became functional.
Methods: Immunohistochemistry was performed on cryopreserved sections of embryonic/fetal human eyes from 7 to 22 weeks' gestation (WG), using antibodies against PAL-E, PV-1 (fenestrations), carbonic anhydrase IV (CA IV), eNOS, and alpha-smooth muscle actin (alphaSMA) and NG2 (two pericyte markers) and the EC marker (CD31). Alkaline phosphatase (APase) enzymatic activity was demonstrated by enzyme histochemistry. Transmission electron microscopy (TEM) was performed on eyes at 11, 14, 16, and 22 WG. Adult human eyes were used as the positive control.
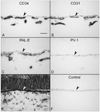
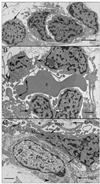
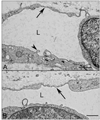
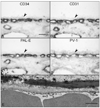
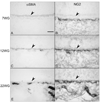
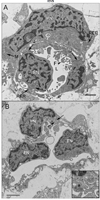
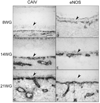
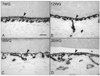
Results: All EC markers were present in the CC by 7 WG. PAL-E, CA IV, and eNOS immunoreactivities and APase activity were present in the CC by 7 to 9 WG. TEM analysis demonstrated how structurally immature this vasculature was, even at 11 WG: no basement membrane, absence of pericytes, and poorly formed lumens that were filled with filopodia. The few fenestrations that were observed were often present within the luminal space in the filopodia. Contiguous fenestrations and significant PV-1 were not observed until 21 to 22 WG. alphaSMA was prominent at 22 WG, and the maturation of pericytes was confirmed by TEM.
Conclusions: It appears that ECs and their precursors express enzymes present in adult CC well before they are structurally mature. Although ECs make tight junctions early in development, contiguous fenestrations and mature pericytes occur much later in development.
Figures



Similar articles
-
Development of the human choriocapillaris.Eye (Lond). 2010 Mar;24(3):408-15. doi: 10.1038/eye.2009.318. Epub 2010 Jan 15. Eye (Lond). 2010. PMID: 20075975 Free PMC article. Review.
-
Role of CD44+ stem cells in mural cell formation in the human choroid: evidence of vascular instability due to limited pericyte ensheathment.Invest Ophthalmol Vis Sci. 2011 Jan 21;52(1):399-410. doi: 10.1167/iovs.10-5403. Invest Ophthalmol Vis Sci. 2011. PMID: 21169526
-
Co-expression of endothelial and neuronal nitric oxide synthases in the developing vasculatures of the human fetal eye.Graefes Arch Clin Exp Ophthalmol. 2012 Jun;250(6):839-48. doi: 10.1007/s00417-012-1969-9. Epub 2012 Mar 14. Graefes Arch Clin Exp Ophthalmol. 2012. PMID: 22411126 Free PMC article.
-
Evidence of hematopoietic differentiation, vasculogenesis and angiogenesis in the formation of human choroidal blood vessels.Exp Eye Res. 2011 May;92(5):361-76. doi: 10.1016/j.exer.2011.02.009. Epub 2011 Feb 24. Exp Eye Res. 2011. PMID: 21354137
-
The NG2 Proteoglycan in Pericyte Biology.Adv Exp Med Biol. 2018;1109:5-19. doi: 10.1007/978-3-030-02601-1_2. Adv Exp Med Biol. 2018. PMID: 30523586 Review.
Cited by
-
Stepwise differentiation and functional characterization of human induced pluripotent stem cell-derived choroidal endothelial cells.Stem Cell Res Ther. 2020 Sep 23;11(1):409. doi: 10.1186/s13287-020-01903-4. Stem Cell Res Ther. 2020. PMID: 32967716 Free PMC article.
-
Increased neovascularization in mice lacking tissue inhibitor of metalloproteinases-3.Invest Ophthalmol Vis Sci. 2011 Aug 3;52(9):6117-23. doi: 10.1167/iovs.10-5899. Invest Ophthalmol Vis Sci. 2011. PMID: 21282576 Free PMC article.
-
Development of the human choriocapillaris.Eye (Lond). 2010 Mar;24(3):408-15. doi: 10.1038/eye.2009.318. Epub 2010 Jan 15. Eye (Lond). 2010. PMID: 20075975 Free PMC article. Review.
-
RESIDUAL CHOROIDAL VESSELS IN ATROPHY CAN MASQUERADE AS CHOROIDAL NEOVASCULARIZATION ON OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY: Introducing a Clinical and Software Approach.Retina. 2018 Jul;38(7):1289-1300. doi: 10.1097/IAE.0000000000001863. Retina. 2018. PMID: 29059100 Free PMC article.
-
Selective Requirements for Vascular Endothelial Cells and Circulating Factors in the Regulation of Retinal Neurogenesis.Front Cell Dev Biol. 2021 Apr 8;9:628737. doi: 10.3389/fcell.2021.628737. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33898420 Free PMC article.
References
-
- Bhutto IA, Lutty GA. The vasculature of choroid. In: Shepro D, D’Amore P, editors. Encyclopedia of Microvasculatures. New York: Elsevier; 2004. pp. 369–374.
-
- Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF, Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

