The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus
- PMID: 19264902
- PMCID: PMC2671357
- DOI: 10.2353/ajpath.2009.080814
The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus
Abstract
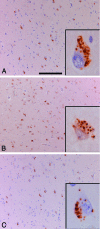
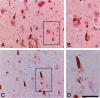
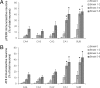
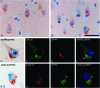
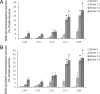
Accumulation of misfolded proteins in the endoplasmic reticulum triggers a cellular stress response called the unfolded protein response (UPR) that protects the cell against the toxic buildup of misfolded proteins. Previously, we reported that UPR activation is increased in Alzheimer's disease (AD) patients. How the UPR relates to the pathological hallmarks of AD is still elusive. In the present study, the involvement of UPR activation in neurofibrillary degeneration in AD was investigated. Immunoreactivity for the phosphorylated UPR activation markers pancreatic ER kinase (pPERK), eukaryotic initiation factor 2alpha, and inositol-requiring enzyme 1alpha was observed in hippocampal neurons associated with granulovacuolar degeneration. The percentage of pPERK-immunoreactive neurons was increased in AD cases compared with nondemented control cases and with the Braak stage for neurofibrillary changes. Although absent from neurofibrillary tangles, pPERK immunoreactivity was most abundant in neurons with diffuse localization of phosphorylated tau protein. Additional analyses showed that pPERK immunoreactivity was associated with ubiquitin and the ubiquitin binding protein p62. A strong co-occurrence of immunoreactivity for both pPERK and glycogen synthase kinase 3beta in neurons was also observed. Together, these data indicate that UPR activation in AD neurons occurs at an early stage of neurofibrillary degeneration and suggest that the prolonged activation of the UPR is involved in both tau phosphorylation and neurodegeneration in AD pathogenesis.
Figures
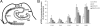

References
-
- Forman MS, Lee VM, Trojanowski JQ. ‘Unfolding’ pathways in neurodegenerative disease. Trends Neurosci. 2003;26:407–410. - PubMed
-
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. - PubMed
-
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. - PubMed
-
- Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. - PubMed
-
- Hoozemans JJ, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol (Berl) 2005;110:165–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

