Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis
- PMID: 19264905
- PMCID: PMC2671354
- DOI: 10.2353/ajpath.2009.080458
Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis
Abstract
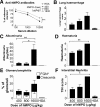
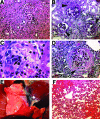
The morbidity burden associated with anti-neutrophil cytoplasmic autoantibody-associated vasculitis is increasing, and many novel biological therapies are now entering the drug development pipeline. There is thus an urgent need to develop a representative animal model to facilitate testing of these agents. We previously examined the effect of antineutrophil cytoplasmic autoantibody on leukocyte-endothelial interactions in WKY rats via immunization with human myeloperoxidase. We now seek to extend this model so that all animals reliably develop crescentic glomerulonephritis and lung hemorrhage. We also wish to investigate whether there is a genetic contribution to vasculitis development in this rat strain. Using escalating doses of human myeloperoxidase, we found that a dose of 1600 microg/kg induced pauci-immune crescentic glomerulonephritis and lung hemorrhage in all immunized animals. We also found that the addition of pertussis toxin and killed Mycobacterium tuberculosis to the adjuvant when immunizing with 400 microg/kg of myeloperoxidase resulted in crescentic glomerulonephritis and lung hemorrhage in all animals. However, when Lewis, Wistar Furth, or Brown Norway rats were immunized using a similar protocol, no animals developed hematuria or glomerulonephritis, despite having identical levels of anti-human myeloperoxidase antibodies. We conclude that, by adjusting the immunization regimen, all WKY rats immunized with myeloperoxidase develop experimental autoimmune vasculitis, thus facilitating future therapeutic studies. The resistance of Lewis rats to experimental autoimmune vasculitis provides a genetic basis for future studies of anti-myeloperoxidase antibody-associated vasculitis.
Figures



References
-
- van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, van der Giessen M, van der Hem GK, The TH. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1:425–429. - PubMed
-
- Niles JL, McCluskey RT, Ahmad MF, Arnaout MA. Wegener’s granulomatosis autoantigen is a novel neutrophil serine proteinase. Blood. 1989;74:1888–1893. - PubMed
-
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. - PubMed
-
- Jennette JC, Hoidal JR, Falk RJ. Specificity of anti-neutrophil cytoplasmic autoantibodies for proteinase 3. Blood. 1990;75:2263–2264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

