Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain
- PMID: 19264913
- PMCID: PMC2671378
- DOI: 10.2353/ajpath.2009.081036
Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain
Abstract
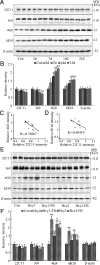
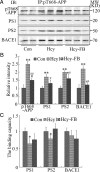
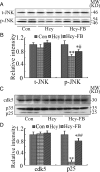
Hyperhomocysteinemia and beta-amyloid (Abeta) overproduction are critical etiological and pathological factors in Alzheimer disease, respectively; however, the intrinsic link between them is still missing. Here, we found that Abeta levels increased and amyloid precursor protein (APP) levels simultaneously decreased in hyperhomocysteinemic rats after a 2-week induction by vena caudalis injection of homocysteine. Concurrently, both the mRNA and protein levels of presenilin-1, a component of gamma-secretase, were elevated, whereas the expression levels of beta-secretase and presenilin-2 were not altered. We also observed that levels of phosphorylated APP at threonine-668, a crucial site facilitating the amyloidogenic cleavage of APP, increased in rats with hyperhomocysteinemia, although the phosphorylation per se did not increase the binding capacity of pT668-APP to the secretases. The enhanced phosphorylation of APP in these rats was not relevant to either c-Jun N-terminal kinase or cyclin-dependent kinase-5. A prominent spatial memory deficit was detected in rats with hyperhomocysteinemia. Simultaneous supplementation of folate and vitamin-B12 attenuated the hyperhomocysteinemia-induced abnormal processing of APP and improved memory. Our data revealed that hyperhomocysteinemia could increase Abeta production through the enhanced expression of gamma-secretase and APP phosphorylation, causing memory deficits that could be rescued by folate and vitamin-B12 treatment in these rats. It is suggested that hyperhomocysteinemia may serve as an upstream factor for increased Abeta production as seen in patients with Alzheimer disease.
Figures




Similar articles
-
Auraptene increases the production of amyloid-β via c-Jun N-terminal kinase-dependent activation of γ-secretase.J Alzheimers Dis. 2015;43(4):1215-28. doi: 10.3233/JAD-141692. J Alzheimers Dis. 2015. PMID: 25147119
-
Phosphorylation of amyloid precursor carboxy-terminal fragments enhances their processing by a gamma-secretase-dependent mechanism.Neurobiol Dis. 2005 Nov;20(2):625-37. doi: 10.1016/j.nbd.2005.05.004. Epub 2005 Jun 3. Neurobiol Dis. 2005. PMID: 15936948
-
Huannao Yicong Formula () regulates γ-secretase activity through APH-1 and PEN-2 gene ragulation pathways in hippocampus of APP/PS1 double transgenic mice.Chin J Integr Med. 2017 Apr;23(4):270-278. doi: 10.1007/s11655-017-2402-3. Epub 2017 Jan 24. Chin J Integr Med. 2017. PMID: 28120208
-
Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
-
gamma-Secretase inhibitors as molecular probes of presenilin function.J Mol Neurosci. 2001 Oct;17(2):199-204. doi: 10.1385/JMN:17:2:199. J Mol Neurosci. 2001. PMID: 11816793 Review.
Cited by
-
Mice deficient in cystathionine beta synthase display increased Dyrk1A and SAHH activities in brain.J Mol Neurosci. 2013 May;50(1):1-6. doi: 10.1007/s12031-012-9835-0. Epub 2012 Jun 15. J Mol Neurosci. 2013. PMID: 22700376
-
Homocysteine Induces Inflammation in Retina and Brain.Biomolecules. 2020 Mar 3;10(3):393. doi: 10.3390/biom10030393. Biomolecules. 2020. PMID: 32138265 Free PMC article.
-
Genetic influence of plasma homocysteine on Alzheimer's disease.Neurobiol Aging. 2018 Feb;62:243.e7-243.e14. doi: 10.1016/j.neurobiolaging.2017.09.033. Epub 2017 Oct 13. Neurobiol Aging. 2018. PMID: 29102475 Free PMC article.
-
The Fox and the Rabbits-Environmental Variables and Population Genetics (1) Replication Problems in Association Studies and the Untapped Power of GWAS (2) Vitamin A Deficiency, Herpes Simplex Reactivation and Other Causes of Alzheimer's Disease.ISRN Neurol. 2011;2011:394678. doi: 10.5402/2011/394678. Epub 2011 Jul 12. ISRN Neurol. 2011. PMID: 22389816 Free PMC article.
-
Mechanistic Link between Vitamin B12 and Alzheimer's Disease.Biomolecules. 2022 Jan 14;12(1):129. doi: 10.3390/biom12010129. Biomolecules. 2022. PMID: 35053277 Free PMC article. Review.
References
-
- Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. - PubMed
-
- Nishitomi K, Sakaguchi G, Horikoshi Y, Gray AJ, Maeda M, Hirata-Fukae C, Becker AG, Hosono M, Sakaguchi I, Minami SS, Nakajima Y, Li HF, Takeyama C, Kihara T, Ota A, Wong PC, Aisen PS, Kato A, Kinoshita N, Matsuoka Y. BACE1 inhibition reduces endogenous abeta and alters APP processing in wild-type mice. J Neurochem. 2006;99:1555–1563. - PubMed
-
- Vassar R. β-Secretase (BACE) as a drug target for Alzheimer’s disease. Adv Drug Del Rev. 2002;54:1589–1602. - PubMed
-
- Iwata N, Saido TC. Amyloid-beta peptide metabolism and Alzheimer’s disease. Nippon Yakurigaku Zasshi. 2003;122:5–14. - PubMed
-
- Selkoe DJ. Amyloid beta-protein and the genetics of Alzheimer’s disease. J Biol Chem. 1996;271:18295–18298. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

