RNA interference-mediated inhibition of erythropoietin receptor expression suppresses tumor growth and invasiveness in A2780 human ovarian carcinoma cells
- PMID: 19264915
- PMCID: PMC2671380
- DOI: 10.2353/ajpath.2009.080592
RNA interference-mediated inhibition of erythropoietin receptor expression suppresses tumor growth and invasiveness in A2780 human ovarian carcinoma cells
Abstract
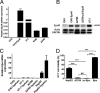
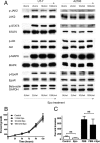
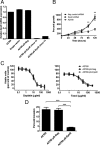
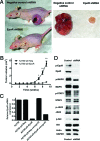
Although recombinant human erythropoietin (rHuEpo) has revolutionized the treatment of anemia, recent clinical trials suggested that rHuEpo use may be associated with decreased survival in cancer patients. Although the expression of erythropoietin (Epo) receptor (EpoR) has been demonstrated in various human cancers, the effect of exogenous Epo on the growth and therapy resistance of EpoR-bearing tumor cells is unclear at present. In the current study, we examined the hypothesis that EpoR may contribute to tumor growth independent of Epo in A2780 human ovarian carcinoma cells. A2780 human ovarian carcinoma cells showed high levels of EpoR expression, but lacked expression of Epo mRNA and biologically active Epo protein under both normoxic and hypoxic conditions. Exogenous Epo did not stimulate EpoR-mediated signaling, proliferation, invasiveness, or resistance to cytotoxic drugs in A2780 cells. In contrast, specific inhibition of EpoR expression using a short hairpin RNA (shRNA) expression plasmid resulted in markedly reduced proliferation and invasiveness in vitro. In addition, inhibition of EpoR expression led to abrogated in vivo ovarian cancer cell growth in a tumor xenograft system and resulted in decreased EpoR signaling. Our findings suggest that EpoR may be constitutively active in some cancer cells in the absence of Epo and provide the first evidence for a potential role of an Epo-independent, EpoR-mediated pathway in the growth of some human cancers.
Figures
Similar articles
-
The erythropoietin/erythropoietin receptor signaling pathway promotes growth and invasion abilities in human renal carcinoma cells.PLoS One. 2012;7(9):e45122. doi: 10.1371/journal.pone.0045122. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028796 Free PMC article.
-
Characterization of erythropoietin receptor and erythropoietin expression and function in human ovarian cancer cells.Int J Cancer. 2008 Jan 15;122(2):274-80. doi: 10.1002/ijc.23068. Int J Cancer. 2008. PMID: 17893874
-
Hypoxia-inducible erythropoietin signaling in squamous dysplasia and squamous cell carcinoma of the uterine cervix and its potential role in cervical carcinogenesis and tumor progression.Am J Pathol. 2003 Jun;162(6):1789-806. doi: 10.1016/S0002-9440(10)64314-3. Am J Pathol. 2003. PMID: 12759237 Free PMC article.
-
[Erythropoietin and drug resistance in breast and ovarian cancers].Ginekol Pol. 2016;87(4):300-4. doi: 10.17772/gp/57817. Ginekol Pol. 2016. PMID: 27321103 Review. Polish.
-
Significance of Erythropoietin Receptor Antagonist EMP9 in Cancers.Vitam Horm. 2017;105:297-310. doi: 10.1016/bs.vh.2017.03.001. Epub 2017 Apr 18. Vitam Horm. 2017. PMID: 28629523 Review.
Cited by
-
Promises and pitfalls in erythopoietin-mediated tissue protection: are nonerythropoietic derivatives a way forward?J Investig Med. 2011 Oct;59(7):1073-82. doi: 10.2310/JIM.0b013e3181ed30bf. J Investig Med. 2011. PMID: 20683348 Free PMC article. Review.
-
Erythropoietin receptor contributes to melanoma cell survival in vivo.Oncogene. 2012 Mar 29;31(13):1649-60. doi: 10.1038/onc.2011.366. Epub 2011 Aug 22. Oncogene. 2012. PMID: 21860424 Free PMC article.
-
Far-western blotting as a solution to the non-specificity of the anti-erythropoietin receptor antibody.Oncol Lett. 2016 Aug;12(2):1575-1580. doi: 10.3892/ol.2016.4782. Epub 2016 Jun 24. Oncol Lett. 2016. PMID: 27446474 Free PMC article.
-
The effect of erythropoietin on normal and neoplastic cells.Biologics. 2012;6:163-89. doi: 10.2147/BTT.S32281. Epub 2012 Jun 27. Biologics. 2012. PMID: 22848149 Free PMC article.
-
The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization.Int J Mol Sci. 2021 Jul 19;22(14):7682. doi: 10.3390/ijms22147682. Int J Mol Sci. 2021. PMID: 34299300 Free PMC article. Review.
References
-
- Jelkmann W. Molecular biology of erythropoietin. Intern Med. 2004;43:649–659. - PubMed
-
- Ebert BL, Bunn HF. Regulation of the erythropoietin gene. Blood. 1999;94:1864–1877. - PubMed
-
- Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. - PubMed
-
- Bittorf T, Buchse T, Sasse T, Jaster R, Brock J. Activation of the transcription factor NF-kappaB by the erythropoietin receptor: structural requirements and biological significance. Cell Signal. 2001;13:673–681. - PubMed
-
- Miura Y, Miura O, Ihle JN, Aoki N. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994;269:29962–29969. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

