Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury
- PMID: 19264952
- PMCID: PMC2696215
- DOI: 10.1152/ajpgi.90464.2008
Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury
Abstract
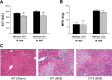
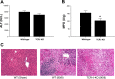
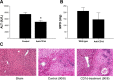
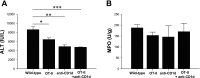
Helper T cells are known to mediate hepatic ischemia/reperfusion (I/R) injury. However, the precise mechanisms and subsets of CD4(+) T cells that contribute to this injury are still controversial. Therefore, we sought to determine the contributions of different CD4(+) T cell subsets during hepatic I/R injury. Wild-type, OT-II, or T cell receptor (TCR)-delta-deficient mice were subjected to 90 min of partial hepatic ischemia followed by 8 h of reperfusion. Additionally, wild-type mice were pretreated with anti-CD1d, -NK1.1, or -IL-2R-alpha antibodies before I/R injury. OT-II mice had diminished liver injury compared with wild-type mice, implicating that antigen-dependent activation of CD4(+) T cells through TCRs is involved in hepatic I/R injury. TCR-delta knockout mice had decreased hepatic neutrophil accumulation, suggesting that gammadelta T cells regulate neutrophil recruitment. We found that natural killer T (NKT) cells, but not NK cells, contribute to hepatic I/R injury via CD1d-dependent activation of their TCRs, as depletion of NKT cells by anti-CD1d antibody or depletion of both NKT cells and NK cells by anti-NK1.1 attenuated liver injury. Although regulatory T cells (Treg) are known to suppress T cell-dependent inflammation, depletion of Treg cells had little effect on hepatic I/R injury. The data suggest that antigen-dependent activation of CD4(+) T cells contributes to hepatic I/R injury. Among the subsets of CD4(+) T cells, it appears that gammadelta T cells contribute to neutrophil recruitment and that NKT cells directly injure the liver. In contrast, NK cells and Treg have little effects on hepatic I/R injury.
Figures

Similar articles
-
Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation.J Exp Med. 2006 Nov 27;203(12):2639-48. doi: 10.1084/jem.20061097. Epub 2006 Nov 6. J Exp Med. 2006. PMID: 17088433 Free PMC article.
-
Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion.Am J Physiol Gastrointest Liver Physiol. 2005 Nov;289(5):G969-76. doi: 10.1152/ajpgi.00223.2005. Epub 2005 Jul 7. Am J Physiol Gastrointest Liver Physiol. 2005. PMID: 16002566
-
Preactivation of NKT cells with alpha-GalCer protects against hepatic ischemia-reperfusion injury in mouse by a mechanism involving IL-13 and adenosine A2A receptor.Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G249-58. doi: 10.1152/ajpgi.00041.2009. Epub 2009 Jun 25. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19556359 Free PMC article.
-
Complexity and function of natural killer T cells with potential application to hepatic transplant survival.Liver Transpl. 2017 Dec;23(12):1589-1592. doi: 10.1002/lt.24950. Liver Transpl. 2017. PMID: 28945950 Free PMC article. Review.
-
Fine tuning a well-oiled machine: Influence of NK1.1 and NKG2D on NKT cell development and function.Int Immunopharmacol. 2013 Oct;17(2):260-6. doi: 10.1016/j.intimp.2013.05.022. Epub 2013 Jun 22. Int Immunopharmacol. 2013. PMID: 23800654 Free PMC article. Review.
Cited by
-
Negative CD4 + TIM-3 signaling confers resistance against cold preservation damage in mouse liver transplantation.Am J Transplant. 2015 Apr;15(4):954-964. doi: 10.1111/ajt.13067. Epub 2015 Feb 12. Am J Transplant. 2015. PMID: 25676534 Free PMC article.
-
Interleukin-22: implications for liver ischemia-reperfusion injury.Transplantation. 2012 Mar 15;93(5):485-92. doi: 10.1097/TP.0b013e3182449136. Transplantation. 2012. PMID: 22262131 Free PMC article.
-
Cell biology of ischemia/reperfusion injury.Int Rev Cell Mol Biol. 2012;298:229-317. doi: 10.1016/B978-0-12-394309-5.00006-7. Int Rev Cell Mol Biol. 2012. PMID: 22878108 Free PMC article. Review.
-
Protective role of heme oxygenase-1 in fatty liver ischemia-reperfusion injury.Med Mol Morphol. 2019 Jun;52(2):61-72. doi: 10.1007/s00795-018-0205-z. Epub 2018 Aug 31. Med Mol Morphol. 2019. PMID: 30171344 Free PMC article. Review.
-
Recipient T cell TIM-3 and hepatocyte galectin-9 signalling protects mouse liver transplants against ischemia-reperfusion injury.J Hepatol. 2015 Mar;62(3):563-72. doi: 10.1016/j.jhep.2014.10.034. Epub 2014 Oct 31. J Hepatol. 2015. PMID: 25450716 Free PMC article.
References
-
- Askenasy N, Kaminitz A, Yarkoni S. Mechanisms of T regulatory cell function. Autoimmun Rev 7: 370–375, 2008. - PubMed
-
- Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science 269: 1727–1730, 1995. - PubMed
-
- Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M. Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol 289: C826–C835, 2005. - PubMed
-
- Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, Lentsch AB. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 289: G969–G976, 2005. - PubMed
-
- Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol 2: 336–345, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

