A P-type ATPase importer that discriminates between essential and toxic transition metals
- PMID: 19264958
- PMCID: PMC2651836
- DOI: 10.1073/pnas.0900666106
A P-type ATPase importer that discriminates between essential and toxic transition metals
Abstract
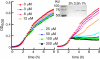
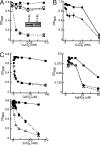
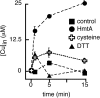
Transition metals, although being essential cofactors in many physiological processes, are toxic at elevated concentrations. Among the membrane-embedded transport proteins that maintain appropriate intracellular levels of transition metals are ATP-driven pumps belonging to the P-type ATPase superfamily. These metal transporters may be differentiated according to their substrate specificities, where the majority of pumps can extrude either silver and copper or zinc, cadmium, and lead. In the present report, we have established the substrate specificities of nine previously uncharacterized prokaryotic transition-metal P-type ATPases. We find that all of the newly identified exporters indeed fall into one of the two above-mentioned categories. In addition to these exporters, one importer, Pseudomonas aeruginosa Q9I147, was also identified. This protein, designated HmtA (heavy metal transporter A), exhibited a different substrate recognition profile from the exporters. In vivo metal susceptibility assays, intracellular metal measurements, and transport experiments all suggest that HmtA mediates the uptake of copper and zinc but not of silver, mercury, or cadmium. The substrate selectivity of this importer ensures the high-affinity uptake of essential metals, while avoiding intracellular contamination by their toxic counterparts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

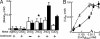

Similar articles
-
Identification of functionally important conserved trans-membrane residues of bacterial PIB -type ATPases.Mol Microbiol. 2014 Feb;91(4):777-89. doi: 10.1111/mmi.12495. Epub 2014 Jan 14. Mol Microbiol. 2014. PMID: 24350798 Free PMC article.
-
A novel Zn (II)/Cd (II)/Pb (II)-translocating PIB-type ATPase mediates metal resistance in Chryseobacterium sp. strain PMSZPI in metal-enriched soil of uranium ore deposit.Int J Biol Macromol. 2025 May;305(Pt 2):141189. doi: 10.1016/j.ijbiomac.2025.141189. Epub 2025 Feb 18. Int J Biol Macromol. 2025. PMID: 39978524
-
Bacterial transition metal P(1B)-ATPases: transport mechanism and roles in virulence.Biochemistry. 2011 Nov 22;50(46):9940-9. doi: 10.1021/bi201418k. Epub 2011 Oct 31. Biochemistry. 2011. PMID: 21999638 Free PMC article. Review.
-
Expression and mutagenesis of ZntA, a zinc-transporting P-type ATPase from Escherichia coli.Biochemistry. 1999 Oct 19;38(42):14109-16. doi: 10.1021/bi9913956. Biochemistry. 1999. PMID: 10529259
-
[Bacterial systems for expelling toxic metals].Rev Latinoam Microbiol. 1998 Jan-Jun;40(1-2):53-71. Rev Latinoam Microbiol. 1998. PMID: 10932735 Review. Spanish.
Cited by
-
Transcriptomics and Functional Analysis of Copper Stress Response in the Sulfate-Reducing Bacterium Desulfovibrio alaskensis G20.Int J Mol Sci. 2022 Jan 26;23(3):1396. doi: 10.3390/ijms23031396. Int J Mol Sci. 2022. PMID: 35163324 Free PMC article.
-
CtpB Facilitates Mycobacterium tuberculosis Growth in Copper-Limited Niches.Int J Mol Sci. 2022 May 20;23(10):5713. doi: 10.3390/ijms23105713. Int J Mol Sci. 2022. PMID: 35628523 Free PMC article.
-
Global Analysis of the Zinc Homeostasis Network in Pseudomonas aeruginosa and Its Gene Expression Dynamics.Front Microbiol. 2021 Oct 8;12:739988. doi: 10.3389/fmicb.2021.739988. eCollection 2021. Front Microbiol. 2021. PMID: 34690984 Free PMC article.
-
Reconsidering the czcD (NiCo) Riboswitch as an Iron Riboswitch.ACS Bio Med Chem Au. 2022 Aug 17;2(4):376-385. doi: 10.1021/acsbiomedchemau.1c00069. Epub 2022 Mar 4. ACS Bio Med Chem Au. 2022. PMID: 35996475 Free PMC article.
-
Heterotrophic nitrification-aerobic denitrification characteristics and zinc-containing wastewater treatment potential of pseudomonas hunanensis SK-4: screening, application, and mechanistic insights.Biodegradation. 2025 Jul 22;36(4):66. doi: 10.1007/s10532-025-10162-0. Biodegradation. 2025. PMID: 40694303
References
-
- Thompson KH, Orvig C. Boon and bane of metal ions in medicine. Science. 2003;300:936–939. - PubMed
-
- Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev. 2003;27:313–339. - PubMed
-
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–337. - PubMed
-
- Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993;3:7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

