Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy
- PMID: 19264968
- PMCID: PMC2660771
- DOI: 10.1073/pnas.0806647106
Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy
Abstract
The poor prognosis of patients with aggressive and invasive cancers combined with toxic effects and short half-life of currently available treatments necessitate development of more effective tumor selective therapies. Mesenchymal stem cells (MSCs) are emerging as novel cell-based delivery agents; however, a thorough investigation addressing their therapeutic potential and fate in different cancer models is lacking. In this study, we explored the engineering potential, fate, and therapeutic efficacy of human MSCs in a highly malignant and invasive model of glioblastoma. We show that engineered MSC retain their "stem-like" properties, survive longer in mice with gliomas than in the normal brain, and migrate extensively toward gliomas. We also show that MSCs are resistant to the cytokine tumor necrosis factor apoptosis ligand (TRAIL) and, when engineered to express secreted recombinant TRAIL, induce caspase-mediated apoptosis in established glioma cell lines as well as CD133-positive primary glioma cells in vitro. Using highly malignant and invasive human glioma models and employing real-time imaging with correlative neuropathology, we demonstrate that MSC-delivered recombinant TRAIL has profound anti-tumor effects in vivo. This study demonstrates the efficacy of diagnostic and therapeutic MSC in preclinical glioma models and forms the basis for developing stem cell-based therapies for different cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

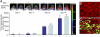
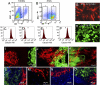


Similar articles
-
Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy.Stem Cells. 2010 Dec;28(12):2217-28. doi: 10.1002/stem.543. Stem Cells. 2010. PMID: 20945331
-
Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells.Neurosurgery. 2009 Sep;65(3):610-24; discussion 624. doi: 10.1227/01.NEU.0000350227.61132.A7. Neurosurgery. 2009. PMID: 19687708
-
Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma.Cancer Res. 2008 Dec 1;68(23):9614-23. doi: 10.1158/0008-5472.CAN-08-0451. Cancer Res. 2008. PMID: 19047138
-
Current status and potential challenges of mesenchymal stem cell-based therapy for malignant gliomas.Stem Cell Res Ther. 2018 Aug 24;9(1):228. doi: 10.1186/s13287-018-0977-z. Stem Cell Res Ther. 2018. PMID: 30143053 Free PMC article. Review.
-
Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour.Int J Mol Sci. 2018 Jul 27;19(8):2188. doi: 10.3390/ijms19082188. Int J Mol Sci. 2018. PMID: 30060445 Free PMC article. Review.
Cited by
-
Migration of mouse-induced pluripotent stem cells to glioma-conditioned medium is mediated by tumor-associated specific growth factors.Oncol Lett. 2011 Mar;2(2):283-288. doi: 10.3892/ol.2011.234. Epub 2011 Jan 14. Oncol Lett. 2011. PMID: 22866078 Free PMC article.
-
The role of mesenchymal stem cells in cancer and prospects for their use in cancer therapeutics.MedComm (2020). 2024 Jul 28;5(8):e663. doi: 10.1002/mco2.663. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39070181 Free PMC article. Review.
-
Mesenchymal Stromal Stem Cell-Derived Microvesicles Enhance Tumor Lysate Pulsed Dendritic Cell Stimulated Autologous T lymphocyte Cytotoxicity.Asian Pac J Cancer Prev. 2018 Jul 27;19(7):1895-1902. doi: 10.22034/APJCP.2018.19.7.1895. Asian Pac J Cancer Prev. 2018. PMID: 30049202 Free PMC article.
-
The Role of Mesenchymal Stem Cells in the Induction of Cancer-Stem Cell Phenotype.Front Oncol. 2022 Feb 17;12:817971. doi: 10.3389/fonc.2022.817971. eCollection 2022. Front Oncol. 2022. PMID: 35251985 Free PMC article. Review.
-
Dynamic assessment of cell viability, proliferation and migration using real time cell analyzer system (RTCA).Cytotechnology. 2015 Mar;67(2):379-86. doi: 10.1007/s10616-014-9692-5. Epub 2014 Jan 19. Cytotechnology. 2015. PMID: 24443077 Free PMC article.
References
-
- Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. - PubMed
-
- Mareschi K, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97:744–754. - PubMed
-
- Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. - PubMed
-
- Corsten MF, et al. MicroRNA-21 knockdown disrupts glioma growth in vivo and displays synergistic cytotoxicity with neural precursor cell delivered S-TRAIL in human gliomas. Cancer Res. 2007;67:8994–9000. - PubMed
-
- Pereboeva L, Komarova S, Mikheeva G, Krasnykh V, Curiel DT. Approaches to utilize mesenchymal progenitor cells as cellular vehicles. Stem Cells. 2003;21:389–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

