Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA
- PMID: 19264974
- PMCID: PMC2689911
- DOI: 10.1164/rccm.200807-1148OC
Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA
Abstract
Rationale: Cigarette smoke (CS) is the primary cause of chronic obstructive pulmonary disease (COPD), an effect that is, in part, due to intense oxidant stress. Clearance of apoptotic cells (efferocytosis) is a critical regulator of lung homeostasis, which is defective in smokers and in patients with COPD, suggesting a role in disease pathogenesis.
Objectives: We hypothesized that CS would impair efferocytosis through oxidant-dependent activation of RhoA, a known inhibitor of this process.
Methods: We investigated the effect of CS on efferocytosis in vivo and ex vivo, using acute, subacute, and long-term mouse exposure models.
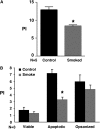
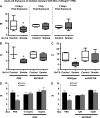
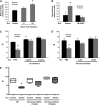
Measurements and main results: Acute and subacute CS exposure suppressed efferocytosis by alveolar macrophages in a dose-dependent, reversible, and cell type-independent manner, whereas more intense CS exposure had an irreversible effect. In contrast, CS did not alter ingestion through the Fc gamma receptor. The inhibitory effect of CS on apoptotic cell clearance depended on oxidants, because the effect was blunted in oxidant-resistant ICR mice, and was prevented by either genetic or pharmacologic antioxidant strategies in vivo and ex vivo. CS inhibited efferocytosis through oxidant-dependent activation of the RhoA-Rho kinase pathway because (1) CS activated RhoA, (2) antioxidants prevented RhoA activation by CS, and (3) inhibitors of the RhoA-Rho kinase pathway reversed the suppressive effect of CS on apoptotic cell clearance in vivo and ex vivo.
Conclusions: These findings advance the hypothesis that impaired efferocytosis may contribute to the pathogenesis of COPD and suggest the therapeutic potential of drugs targeting the RhoA-Rho kinase pathway.
Figures



Similar articles
-
Acute and chronic alcohol exposure impair the phagocytosis of apoptotic cells and enhance the pulmonary inflammatory response.Alcohol Clin Exp Res. 2010 Oct;34(10):1723-32. doi: 10.1111/j.1530-0277.2010.01259.x. Epub 2010 Jul 1. Alcohol Clin Exp Res. 2010. PMID: 20608904 Free PMC article.
-
Neuroendocrine signaling via the serotonin transporter regulates clearance of apoptotic cells.J Biol Chem. 2014 Apr 11;289(15):10466-10475. doi: 10.1074/jbc.M113.482299. Epub 2014 Feb 25. J Biol Chem. 2014. PMID: 24570000 Free PMC article.
-
Cigarette smoke induces endoplasmic reticulum stress and suppresses efferocytosis through the activation of RhoA.Sci Rep. 2020 Jul 28;10(1):12620. doi: 10.1038/s41598-020-69610-x. Sci Rep. 2020. PMID: 32724133 Free PMC article.
-
Efferocytosis and lung disease.Chest. 2013 Jun;143(6):1750-1757. doi: 10.1378/chest.12-2413. Chest. 2013. PMID: 23732585 Free PMC article. Review.
-
Statin-regulated phagocytosis and efferocytosis in physiological and pathological conditions.Pharmacol Ther. 2022 Oct;238:108282. doi: 10.1016/j.pharmthera.2022.108282. Epub 2022 Sep 18. Pharmacol Ther. 2022. PMID: 36130624 Review.
Cited by
-
Genetic evidence linking lung cancer and COPD: a new perspective.Appl Clin Genet. 2011 Jul 18;4:99-111. doi: 10.2147/TACG.S20083. Print 2011. Appl Clin Genet. 2011. PMID: 23776371 Free PMC article.
-
TLR9 expression is required for the development of cigarette smoke-induced emphysema in mice.Am J Physiol Lung Cell Mol Physiol. 2016 Jul 1;311(1):L154-66. doi: 10.1152/ajplung.00073.2016. Epub 2016 Jun 10. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27288485 Free PMC article.
-
A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation.PLoS One. 2013;8(3):e58258. doi: 10.1371/journal.pone.0058258. Epub 2013 Mar 6. PLoS One. 2013. PMID: 23484005 Free PMC article.
-
Camellia sinensis L. Alleviates Pulmonary Inflammation Induced by Porcine Pancreas Elastase and Cigarette Smoke Extract.Antioxidants (Basel). 2022 Aug 28;11(9):1683. doi: 10.3390/antiox11091683. Antioxidants (Basel). 2022. PMID: 36139757 Free PMC article.
-
Second hand smoke and COPD: lessons from animal studies.Front Physiol. 2013 Feb 27;4:30. doi: 10.3389/fphys.2013.00030. eCollection 2013. Front Physiol. 2013. PMID: 23450717 Free PMC article.
References
-
- MacNee W. Oxidants/antioxidants and COPD. Chest 2000;117(5, Suppl 1):303S–317S. - PubMed
-
- Rahman I, Li XY, Donaldson K, Harrison DJ, MacNee W. Glutathione homeostasis in alveolar epithelial cells in vitro and lung in vivo under oxidative stress. Am J Physiol 1995;269:L285–L292. - PubMed
-
- Li XY, Donaldson K, Rahman I, MacNee W. An investigation of the role of glutathione in increased epithelial permeability induced by cigarette smoke in vivo and in vitro. Am J Respir Crit Care Med 1994;149:1518–1525. - PubMed
-
- Cavarra E, Bartalesi B, Lucattelli M, Fineschi S, Lunghi B, Gambelli F, Ortiz LA, Martorana PA, Lungarella G. Effects of cigarette smoke in mice with different levels of α1-proteinase inhibitor and sensitivity to oxidants. Am J Respir Crit Care Med 2001;164:886–890. - PubMed
-
- Bartalesi B, Cavarra E, Fineschi S, Lucattelli M, Lunghi B, Martorana PA, Lungarella G. Different lung responses to cigarette smoke in two strains of mice sensitive to oxidants. Eur Respir J 2005;25:15–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

