Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades
- PMID: 19265036
- PMCID: PMC3085025
- DOI: 10.1161/CIRCRESAHA.108.192765
Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades
Abstract
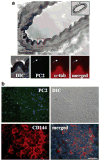
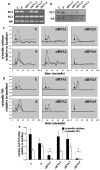
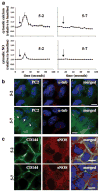
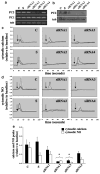
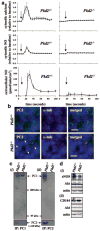
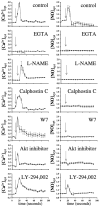
Cardiovascular complications such as hypertension are a continuous concern in patients with autosomal dominant polycystic kidney disease (ADPKD). The PKD2 encoding for polycystin-2 is mutated in approximately 15% of ADPKD patients. Here, we show that polycystin-2 is localized to the cilia of mouse and human vascular endothelial cells. We demonstrate that the normal expression level and localization of polycystin-2 to cilia is required for the endothelial cilia to sense fluid shear stress through a complex biochemical cascade, involving calcium, calmodulin, Akt/PKB, and protein kinase C. In response to fluid shear stress, mouse endothelial cells with knockdown or knockout of Pkd2 lose the ability to generate nitric oxide (NO). Consistent with mouse data, endothelial cells generated from ADPKD patients do not show polycystin-2 in the cilia and are unable to sense fluid flow. In the isolated artery, we further show that ciliary polycystin-2 responds specifically to shear stress and not to mechanical stretch, a pressurized biomechanical force that involves purinergic receptor activation. We propose a new role for polycystin-2 in transmitting extracellular shear stress to intracellular NO biosynthesis. Thus, aberrant expression or localization of polycystin-2 to cilia could promote high blood pressure because of inability to synthesize NO in response to an increase in shear stress (blood flow).
Figures

References
-
- Boucher C, Sandford R. Autosomal dominant polycystic kidney disease (ADPKD, MIM 173900, PKD1 and PKD2 genes, protein products known as polycystin-1 and polycystin-2) Eur J Hum Genet. 2004;12:347–354. - PubMed
-
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. - PubMed
-
- Hateboer N, Veldhuisen B, Peters D, Breuning MH, San-Millan JL, Bogdanova N, Coto E, van Dijk MA, Afzal AR, Jeffery S, Saggar-Malik AK, Torra R, Dimitrakov D, Martinez I, de Castro SS, Krawczak M, Ravine D. Location of mutations within the PKD2 gene influences clinical outcome. Kidney Int. 2000;57:1444–1451. - PubMed
-
- Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353:103–107. - PubMed
-
- Wu G, Markowitz GS, Li L, D’Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H, Jr, Kucherlapati R, Edelmann W, Somlo S. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet. 2000;24:75–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

