ERK5 knockdown generates mouse leukemia cells with low MHC class I levels that activate NK cells and block tumorigenesis
- PMID: 19265117
- PMCID: PMC6311211
- DOI: 10.4049/jimmunol.0803006
ERK5 knockdown generates mouse leukemia cells with low MHC class I levels that activate NK cells and block tumorigenesis
Abstract
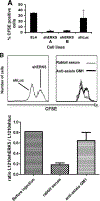
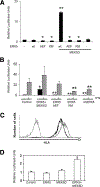
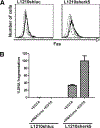
Tumor cell-based vaccines are currently used in clinical trails, but they are in general poorly immunogenic because they are composed of cell extracts or apoptotic cells. Live tumor cells should be much better Ags provided that they are properly processed by the host immune system. We show herein that stable expression of a small hairpin RNA for ERK5 (shERK5) decreases ERK5 levels in human and mouse leukemic cells and leads to their elimination by NK cells in vivo. The shERK5 cells show down-regulation of MHC class I expression at the plasma membrane. Accordingly, ectopic activation of the ERK5 pathway induces MHC class I gene expression. Coinjection of shERK5-expressing cells into the peritoneum diminishes survival of engrafted wild-type tumor cells. Moreover, s.c. injection of shERK5-expressing cells strongly diminishes tumor development by wild-type cells. Our results show that shERK5 expression in leukemia cells effectively attenuates their tumor activity and allows their use as a tumor cell-based vaccine.
Figures




References
-
- Dunn GP, Koebel CM, and Schreiber RD 2006. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol 6: 836–848. - PubMed
-
- Nguyen van Binh P, and Duc HT 2006. Analyses and perspectives in cancer immunotherapy. Biomed. Pharmacother 60: 621–628. - PubMed
-
- Berd D, Maguire HC Jr., McCue P, and Mastrangelo MJ 1990. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J. Clin. Oncol 8: 1858–1867. - PubMed
-
- Ronchetti A, Rovere P, Iezzi G, Galati G, Heltai S, Protti MP,Garancini MP, Manfredi AA, Rugarli C, and Bellone M 1999. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J. Immunol 163: 130–136. - PubMed
-
- Dzik WH 2003. Apoptosis, TGFβ and transfusion-related immunosuppression: biologic versus clinical effects. Transfus. Apher. Sci 29: 127–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

