A central role for induced regulatory T cells in tolerance induction in experimental colitis
- PMID: 19265124
- PMCID: PMC2763205
- DOI: 10.4049/jimmunol.0802535
A central role for induced regulatory T cells in tolerance induction in experimental colitis
Abstract
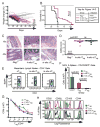
In addition to thymus-derived or natural T regulatory (nT(reg)) cells, a second subset of induced T regulatory (iT(reg)) cells arises de novo from conventional CD4(+) T cells in the periphery. The function of iT(reg) cells in tolerance was examined in a CD45RB(high)CD4(+) T cell transfer model of colitis. In situ-generated iT(reg) cells were similar to nT(reg) cells in their capacity to suppress T cell proliferation in vitro and their absence in vivo accelerated bowel disease. Treatment with nT(reg) cells resolved the colitis, but only when iT(reg) cells were also present. Although iT(reg) cells required Foxp3 for suppressive activity and phenotypic stability, their gene expression profile was distinct from the established nT(reg) "genetic signature," indicative of developmental and possibly mechanistic differences. These results identified a functional role for iT(reg) cells in vivo and demonstrated that both iT(reg) and nT(reg) cells can act in concert to maintain tolerance.
Figures



References
-
- Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. - PubMed
-
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. - PubMed
-
- Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting edge: IL-2 is essential for TGF-β-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

