Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice
- PMID: 19265127
- PMCID: PMC4082659
- DOI: 10.4049/jimmunol.0803052
Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice
Abstract
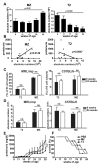
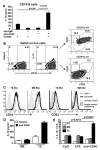
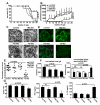
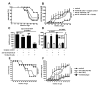
We have previously reported that IL-10(+) regulatory B cells, known to play an important role in controlling autoimmunity and inflammatory disorders, are contained within the transitional 2 immature (T2) B cell pool (T2 Bregs). Therapeutic strategies facilitating their enrichment or enhancing their suppressive activity are highly attractive. In this study, we report that agonistic anti-CD40 specifically targets T2 B cells and enriches Bregs upon short-term in vitro culture. Although transfer of unmanipulated T2 B cells, isolated from mice with established lupus, failed to confer protection to diseased mice, transfer of in vitro anti-CD40-generated T2 B cells (T2-like-Bregs) significantly improved renal disease and survival by an IL-10-dependent mechanism. T2-like-Bregs readily accumulated in the spleen after transfer, suppressed Th1 responses, induced the differentiation of IL-10(+)CD4(+)T cells, and conveyed a regulatory effect to CD4(+)T cells. In addition, in vivo administration of agonistic anti-CD40, currently on trial for the treatment of cancer, halted and reversed established lupus. Taken together, our results suggest a novel cellular approach for the amelioration of experimental lupus.
Figures



References
-
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. - PubMed
-
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. - PubMed
-
- Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29:34–40. - PubMed
-
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

