ESAT-6 inhibits production of IFN-gamma by Mycobacterium tuberculosis-responsive human T cells
- PMID: 19265145
- PMCID: PMC5488288
- DOI: 10.4049/jimmunol.0803579
ESAT-6 inhibits production of IFN-gamma by Mycobacterium tuberculosis-responsive human T cells
Abstract
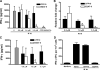
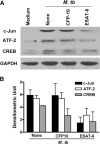
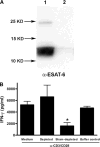
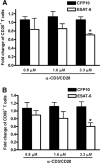
The Mycobacterium tuberculosis early secreted Ag of 6 kDa (ESAT-6) is a potent Ag for human T cells and is a putative vaccine candidate. However, ESAT-6 also contributes to virulence in animal models, mediates cellular cytolysis, and inhibits IL-12 production by mononuclear phagocytes. We evaluated the effects of ESAT-6 and its molecular chaperone, culture filtrate protein of 10 kDa (CFP10), on the capacity of human T cells to produce IFN-gamma and proliferate in response to TCR activation. Recombinant ESAT-6, but not CFP10, markedly inhibited IFN-gamma production by T cells stimulated with M. tuberculosis or with the combination of anti-CD3 and anti-CD28, in a dose-dependent manner. ESAT-6 also inhibited T cell production of IL-17 and TNF-alpha but not IL-2. Preincubation of ESAT-6 with CFP10 under conditions that favor dimer formation did not affect inhibition of IFN-gamma. ESAT-6 decreased IFN-gamma transcription and reduced expression of the transcription factors, ATF-2 and c-Jun, which normally bind to the IFN-gamma proximal promoter and stimulate mRNA expression. ESAT-6 inhibited T cell IFN-gamma secretion through mechanisms that did not involve cellular cytotoxicity or apoptosis. ESAT-6, but not CFP10, bound to T cells and inhibited expression of early activation markers without reducing activation of ZAP70. We conclude that ESAT-6 directly inhibits human T cell responses to mycobacterial Ags by affecting TCR signaling pathways downstream of ZAP70.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

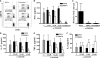
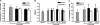


Similar articles
-
Mycobacterium tuberculosis ESX-1 system-secreted protein ESAT-6 but not CFP10 inhibits human T-cell immune responses.Tuberculosis (Edinb). 2009 Dec;89 Suppl 1:S74-6. doi: 10.1016/S1472-9792(09)70017-4. Tuberculosis (Edinb). 2009. PMID: 20006311
-
The Mycobacterium tuberculosis early secreted antigenic target of 6 kDa inhibits T cell interferon-γ production through the p38 mitogen-activated protein kinase pathway.J Biol Chem. 2011 Jul 8;286(27):24508-18. doi: 10.1074/jbc.M111.234062. Epub 2011 May 17. J Biol Chem. 2011. PMID: 21586573 Free PMC article.
-
ESAT-6 Targeting to DEC205+ Antigen Presenting Cells Induces Specific-T Cell Responses against ESAT-6 and Reduces Pulmonary Infection with Virulent Mycobacterium tuberculosis.PLoS One. 2015 Apr 27;10(4):e0124828. doi: 10.1371/journal.pone.0124828. eCollection 2015. PLoS One. 2015. PMID: 25915045 Free PMC article.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis.Vaccine. 2014 Feb 3;32(6):712-6. doi: 10.1016/j.vaccine.2013.11.065. Epub 2013 Dec 2. Vaccine. 2014. PMID: 24300592 Review.
Cited by
-
Early Secreted Antigenic Target of 6-kDa of Mycobacterium tuberculosis Stimulates IL-6 Production by Macrophages through Activation of STAT3.Sci Rep. 2017 Jan 20;7:40984. doi: 10.1038/srep40984. Sci Rep. 2017. PMID: 28106119 Free PMC article.
-
An integrative and comprehensive analysis of blood transcriptomes combined with machine learning models reveals key signatures for tuberculosis diagnosis and risk stratification.Front Microbiol. 2025 May 26;16:1546770. doi: 10.3389/fmicb.2025.1546770. eCollection 2025. Front Microbiol. 2025. PMID: 40491837 Free PMC article.
-
Mycobacterium tuberculosis and host interactions in the manifestation of tuberculosis.J Clin Tuberc Other Mycobact Dis. 2024 Jun 14;36:100458. doi: 10.1016/j.jctube.2024.100458. eCollection 2024 Aug. J Clin Tuberc Other Mycobact Dis. 2024. PMID: 38983441 Free PMC article. Review.
-
The stringent response and Mycobacterium tuberculosis pathogenesis.Pathog Dis. 2018 Jul 1;76(5):fty054. doi: 10.1093/femspd/fty054. Pathog Dis. 2018. PMID: 29947752 Free PMC article. Review.
-
The effect of C. burnetii infection on the cytokine response of PBMCs from pregnant goats.PLoS One. 2014 Oct 3;9(10):e109283. doi: 10.1371/journal.pone.0109283. eCollection 2014. PLoS One. 2014. PMID: 25279829 Free PMC article.
References
-
- Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. J Am Med Assoc. 1995;273:220–226. - PubMed
-
- Andersen P, Andersen AB, Sorensen AL, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. - PubMed
-
- Dietrich J, Billeskov R, Doherty TM, Andersen P. Synergistic effect of bacillus calmette guerin and a tuberculosis subunit vaccine in cationic liposomes: increased immunogenicity and protection. J Immunol. 2007;178:3721–3730. - PubMed
-
- Pym AS, Brodin P, Majlessi L, Brosch R, Demangel C, Williams A, Griffiths KE, Marchal G, Leclerc C, Cole ST. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med. 2003;9:533–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

