Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration
- PMID: 19265165
- PMCID: PMC2661207
- DOI: 10.4049/jimmunol.0803330
Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration
Abstract
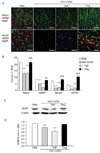
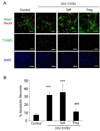
HIV-1-associated neurocognitive impairments are intrinsically linked to microglial immune activation, persistent viral infection, and inflammation. In the era of antiretroviral therapy, more subtle cognitive impairments occur without adaptive immune compromise. We posit that adaptive immunity is neuroprotective, serving in both the elimination of infected cells through CD8(+) cytotoxic T cell activities and the regulation of neuroinflammatory responses of activated microglia. For the latter, little is known. Thus, we studied the neuromodulatory effects of CD4(+) regulatory T cells (Treg; CD4(+)CD25(+)) or effector T cells in HIV-1-associated neurodegeneration. A newly developed HIV-1 encephalitis mouse model was used wherein murine bone marrow-derived macrophages are infected with a full-length HIV-1(YU2)/vesicular stomatitis viral pseudotype and injected into basal ganglia of syngeneic immunocompetent mice. Adoptive transfer of CD3-activated Treg attenuated astrogliosis and microglia inflammation with concomitant neuroprotection. Moreover, Treg-mediated anti-inflammatory activities and neuroprotection were associated with up-regulation of brain-derived neurotrophic factor and glial cell-derived neurotrophic factor expression and down-regulation of proinflammatory cytokines, oxidative stress, and viral replication. Effector T cells showed contrary effects. These results, taken together, demonstrate the importance of Treg in disease control and raise the possibility of their utility for therapeutic strategies.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures





Similar articles
-
Brain ingress of regulatory T cells in a murine model of HIV-1 encephalitis.J Neuroimmunol. 2011 Jan;230(1-2):33-41. doi: 10.1016/j.jneuroim.2010.08.014. Epub 2010 Sep 16. J Neuroimmunol. 2011. PMID: 20846730 Free PMC article.
-
Neuroregulatory events follow adaptive immune-mediated elimination of HIV-1-infected macrophages: studies in a murine model of viral encephalitis.J Immunol. 2004 Jun 15;172(12):7610-7. doi: 10.4049/jimmunol.172.12.7610. J Immunol. 2004. PMID: 15187141
-
Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis.J Immunol. 2002 Apr 15;168(8):3941-9. doi: 10.4049/jimmunol.168.8.3941. J Immunol. 2002. PMID: 11937550
-
HIV-1-infection in the CNS. A pathogenesis of some neurological syndromes in the light of recent investigations.Folia Neuropathol. 1998;36(4):211-6. Folia Neuropathol. 1998. PMID: 10079602 Review.
-
Role of immune activation and cytokine expression in HIV-1-associated neurologic diseases.Adv Neuroimmunol. 1995;5(3):335-58. doi: 10.1016/0960-5428(95)00012-q. Adv Neuroimmunol. 1995. PMID: 8748077 Review.
Cited by
-
Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior.Neuropsychopharmacology. 2012 Jan;37(1):137-62. doi: 10.1038/npp.2011.205. Epub 2011 Sep 14. Neuropsychopharmacology. 2012. PMID: 21918508 Free PMC article. Review.
-
Regulatory T cell in stroke: a new paradigm for immune regulation.Clin Dev Immunol. 2013;2013:689827. doi: 10.1155/2013/689827. Epub 2013 Aug 4. Clin Dev Immunol. 2013. PMID: 23983771 Free PMC article. Review.
-
Brain ingress of regulatory T cells in a murine model of HIV-1 encephalitis.J Neuroimmunol. 2011 Jan;230(1-2):33-41. doi: 10.1016/j.jneuroim.2010.08.014. Epub 2010 Sep 16. J Neuroimmunol. 2011. PMID: 20846730 Free PMC article.
-
Rodent models for HIV-associated neurocognitive disorders.Trends Neurosci. 2012 Mar;35(3):197-208. doi: 10.1016/j.tins.2011.12.006. Epub 2012 Feb 1. Trends Neurosci. 2012. PMID: 22305769 Free PMC article. Review.
-
Enhanced human immunodeficiency virus Type 1 expression and neuropathogenesis in knockout mice lacking Type I interferon responses.J Neuropathol Exp Neurol. 2014 Jan;73(1):59-71. doi: 10.1097/NEN.0000000000000026. J Neuropathol Exp Neurol. 2014. PMID: 24335529 Free PMC article.
References
-
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. - PMC - PubMed
-
- Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV. Int Rev Psychiatry. 2008;20:3–13. - PubMed
-
- Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, Heaton RK. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59:1563–1567. - PubMed
-
- Gisslen M, Hagberg L, Rosengren L, Brew BJ, Cinque P, Spudich S, Price RW. Defining and evaluating HIV-related neurodegenerative disease and its treatment targets: a combinatorial approach to use of cerebrospinal fluid molecular biomarkers. J Neuroimmune Pharmacol. 2007;2:112–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS043985/NS/NINDS NIH HHS/United States
- P01MH64570/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- P01 NS31492/NS/NINDS NIH HHS/United States
- 2R01 NS034239/NS/NINDS NIH HHS/United States
- P20RR 15635/RR/NCRR NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R37 NS036126/NS/NINDS NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- P01 NS43985/NS/NINDS NIH HHS/United States
- P20 RR015635/RR/NCRR NIH HHS/United States
- 2R37 NS36126/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

