Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice
- PMID: 19265174
- PMCID: PMC10283344
- DOI: 10.1165/rcmb.2008-0182OC
Role of proinflammatory cytokines IL-18 and IL-1beta in bleomycin-induced lung injury in humans and mice
Abstract
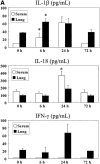
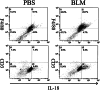
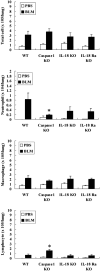
Administration of several chemotherapeutic drugs, such as bleomycin, busulfan, and gefitinib, often induces lethal lung injury. However, the precise mechanisms responsible for this drug-induced lung injury are still unclear. In the present study, we examined the role of the proinflammatory cytokines IL-18 and IL-1beta in the mechanism of bleomycin-induced lung injury. We performed immunohistochemical analysis of IL-18 and IL-18 receptor (R) alpha chain expression in the lungs of five patients with bleomycin-induced lethal lung injury. Enhanced expression of both IL-18 and IL-18Ralpha was observed in the lungs of all five patients with bleomycin-induced lung injury. To support the data obtained from patient samples, the levels of IL-1beta and IL-18 mRNA and protein, pulmonary inflammation, and lung fibrosis were examined in mouse models of bleomycin-induced lung injury. Intravenous administration of bleomycin induced the expression of IL-1beta and IL-18 in the serum and lungs of wild-type C57BL/6 mice. IL-18-producing F4/80(+) neutrophils, but not CD3(+) T cells, were greatly increased in the lungs of treated mice. Moreover, bleomycin-induced lung injury was significantly attenuated in caspase-1(-/-), IL-18(-/-), and IL-18Ralpha(-/-) mice in comparison with control mice. Thus, our results provide evidence for an important role of IL-1beta and IL-18 in chemotherapy-induced lung injury.
Figures








Similar articles
-
B cell activating factor is central to bleomycin- and IL-17-mediated experimental pulmonary fibrosis.J Autoimmun. 2015 Jan;56:1-11. doi: 10.1016/j.jaut.2014.08.003. Epub 2014 Oct 23. J Autoimmun. 2015. PMID: 25441030
-
Signaling through the interleukin-18 receptor α attenuates inflammation in cisplatin-induced acute kidney injury.Kidney Int. 2012 Oct;82(8):892-902. doi: 10.1038/ki.2012.226. Epub 2012 Jun 6. Kidney Int. 2012. PMID: 22673883
-
Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin-induced lung injury.J Immunol. 2011 Jan 15;186(2):1097-106. doi: 10.4049/jimmunol.1002907. Epub 2010 Dec 13. J Immunol. 2011. PMID: 21149612 Free PMC article.
-
[Heme oxygenase-1 (HO-1) gene knockout affects the balance of lung immune cell composition and aggravates inflammatory injury in lung tissues of LPS-induced acute lung injury (ALI) mice].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024 Apr;40(4):296-302. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024. PMID: 38710513 Chinese.
-
[Interventional effects of BAY11-7082 on lung inflammatory response at the early stage and acute lung injury of rats with severe burns].Zhonghua Shao Shang Za Zhi. 2018 Feb 20;34(2):88-95. doi: 10.3760/cma.j.issn.1009-2587.2018.02.006. Zhonghua Shao Shang Za Zhi. 2018. PMID: 29973026 Chinese.
Cited by
-
Modulation of bleomycin-induced lung fibrosis by pegylated hyaluronidase and dopamine receptor antagonist in mice.PLoS One. 2015 Apr 30;10(4):e0125065. doi: 10.1371/journal.pone.0125065. eCollection 2015. PLoS One. 2015. PMID: 25927611 Free PMC article.
-
Maraviroc-Mediated Lung Protection following Trauma-Hemorrhagic Shock.Biomed Res Int. 2016;2016:5302069. doi: 10.1155/2016/5302069. Epub 2016 Jul 31. Biomed Res Int. 2016. PMID: 27556035 Free PMC article.
-
In vitro exposure of precision-cut lung slices to 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole lysylamide dihydrochloride (NSC 710305, Phortress) increases inflammatory cytokine content and tissue damage.Toxicol Sci. 2013 Feb;131(2):470-9. doi: 10.1093/toxsci/kfs319. Epub 2012 Nov 9. Toxicol Sci. 2013. PMID: 23143926 Free PMC article.
-
Gene expression in obliterative bronchiolitis-like lesions in 2,3-pentanedione-exposed rats.PLoS One. 2015 Feb 24;10(2):e0118459. doi: 10.1371/journal.pone.0118459. eCollection 2015. PLoS One. 2015. PMID: 25710175 Free PMC article.
-
Artesunate Inhibits Renal Ischemia-Reperfusion-Mediated Remote Lung Inflammation Through Attenuating ROS-Induced Activation of NLRP3 Inflammasome.Inflammation. 2018 Aug;41(4):1546-1556. doi: 10.1007/s10753-018-0801-z. Inflammation. 2018. PMID: 29730819
References
-
- Sleijfer S. Bleomycin-induced pneumonitis. Chest 2001;120:617–624. - PubMed
-
- Scheule RK, Perkins RC, Hamilton R, Holian A. Bleomycin stimulation of cytokine secretion by the human alveolar macrophage. Am J Physiol 1992;262:L386–L391. - PubMed
-
- Smith RE, Strieter RM, Phan SH, Lukacs NW, Huffnagle GB, Wilke CA, Burdick MD, Lincoln P, Evanoff H, Kunkel SL. Production and function of murine macrophage inflammatory protein-1 alpha in bleomycin-induced lung injury. J Immunol 1994;153:4704–4712. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

